EFFECTS OF STRYCHNINE WITH SPECIAL REFERENCE TO SPINAL AFFERENT FIBERS12 [128]
P.D. Wall, W.S. McCulloch, J.Y. Lettvin and W.H. Pitts
##
Thou art man and canst abide a truth Tho bitter — Tennyson
In 1809 Magendie(1) said in a lecture to the Institute of France: “an entire family of vegetables (the bitter strychnos) has the singular property of exciting strongly the spinal marrow.” Since that time, the dramatic property of strychnine of inducing convulsions has enjoyed the attention of many research workers. This interest was exaggerated by the development of the technique of “strychnine neuronography” by Dusser de Barenne. The localized convulsions generated in the region of locally applied strychnine were used to follow first-order pathways. Attempts have been made to locate those structures within the central nervous system on which strychnine acts and to understand the way in which the convulsive activity is generated. Much of this work has been reviewed by two major contributors to our knowledge of strychnine; Dusser de Barenne (2, 3) and Bremer (4, 5).
Effect on Different Species
The administration of strychnine has a similar effect on all mammalian species so far investigated. The very low dosage used in strychnine “tonics” has no observable pharmacological effect other than autonomic effects, limited mainly to the gastrointestinal system, resulting from the extremely bitter taste of dilute solutions of strychnine. This effect differs in no way from other bitter solutions, such as quinine or quassia. The first toxic signs are hyperexcitability, disorganization of the spinal reflexes, and ataxia. An increase in dosage results in generalized convulsions, which are exaggerated by any form of sensory stimulation. A further increase in dosage kills the animal because of the continuous convulsions. With very high doses death occurs from depression of the heart and paralysis of the neuromuscular junctions. Thus it is not possible to protect the animal from the lethal effects of the very high doses of strychnine by control of the convulsions by barbiturate administration, which does reduce the lethal effect of lower doses. Studies by Munch, Garlough, and Ward (6) show considerable variations between various mammalian species in the lethal convulsion, and hyperexcitability dosage. Guinea pigs and rats require a rather high dose to show the effects, as compared with mice, which are in turn more resistant than dogs, cats, and rabbits, whose lethal dose of hypodermically administered strychnine sulphate is 0.3-0.5 mgm per kilo.
Frogs and fish show a series of effects similar to that of mammals on administration of strychnine (7). But the invertebrates have a quite different response, in which the peripheral actions of strychnine predominate. It was observed in 1882 by Luchsinger that lobsters become red and paralyzed and die without showing any signs of convulsions or hyperexcitability. Bonnet (8) confirmed this effect on the crayfish, and showed that although a reversible paralysis could be produced by low doses, death of the animal always followed within 24 hours. Of other invertebrates, Viehoever and Cohen (9) report similar results on the fresh water prawn, Palaemonetes. However, Daphnia is said to suffer from convulsions before the heart rate drops, and paralysis and death follow. House flies suffer from violent tremors of the legs, followed by paralysis and death.
General Effects on the Central Nervous System
Local or systemic application of adequate concentrations of strychnine to the central nervous system of mammals results in roughly synchronized repetitive firing of the cells. The rate of firing depends on the amount of background activity, the type of cell, the amount and nature of sensory stimulation, and the presence of other drugs. However, certain exceptions appear to the generalization that strychnine produces such activity in all areas. Dow (9) showed that local application to cerebellar cortex failed to produce the spiking activity that can be produced from all parts of the cerebral cortex. Frankenhaeuser (10) failed to find strychnine spikes generated in tracts running from the olfactory bulb or in the vagus nerve. Wall and Horwitz (11) showed that spikes were not generated in the Edinger-Westphal nucleus or in the lateral horn cells of the spinal cord. The failure of the appearance of spiking is, of course, not significant unless it is certain that adequate concentrations have reached the structures examined. The authors, as reported below, had considerable difficulty in evoking spikes from the nuclei of Goll and Burdach by local application on the surface, but when strychnine was injected directly into the nuclei, spiking began almost immediately. The white matter on the surface of the nucleus evidently presented a sufficient barrier to penetration to the region of the cell bodies. A barrier to penetration may explain the failure, reported by some surgeons, to evoke strychnine spikes from the human cerebral cortex. It was shown by Wall and Horwitz that if the lateral horn cells that were not responding were stimulated by a single electric pulse, the cells subsequently generated a number of spikes before lapsing into inactivity. This suggests that an artificial increase in the activity of apparently unaffected cells may result in the typical spiking activity. A further possible reason for the apparent failure of the spike response could be that the activity of inhibitory and excitatory systems may be increased. An example of this is seen in the work of Terzuolo (12), which shows the cerebellar inhibition of strychnine tetanus in the spinal cord. An example of a strychninized area suppressing spontaneous activity in another is seen in the work of Dusser de Barenne and McCulloch (13). It is therefore possible that failure of response may reflect the powerful inhibition of the cells by another firing system. This, of course, may be unlikely in local strychninizations, where no spikes can be recorded at the site of strychninization, but must be considered in the case of systemic administration of the drug.
It is interesting to note the work of Busquet and Vischniac (14), who showed that during the postconvulsive period in rabbits no apparent effects follow a second dose which was previously sufficient to produce convulsions. The rate of firing of cells during the spike was measured by Adrian and Moruzzi (15), who recorded from the pyramidal tract. Bursts of impulses contained from 1080 impulses, with intervals between 0.6 and 4 milliseconds. The report of this high frequency in single units should perhaps be taken with caution, since it is no longer certain the authors were, in fact, recording from single units within the pyramidal tract. Jalavisto (16), recording from single motor nerve fibers in the frog, found 3.6 milliseconds to be the shortest interval between impulses during a burst.
Recently, most surprising details on the generation of a spike in the cortex have been published by Li (17). Local application of strychnine to a single cortical neuron through a micropipette produced either continuous or “burst” firing. With application of strychnine to the cortical surface, some cells fired continuously, being interrupted at the time of the spikes; others fired in bursts at the time of the spikes. Thus individual cells within the strychninized cortex fall into two types. Local strychnine on the cortex may produce not only spikes but a “spike and wave” complex. Li found that while the spike was associated with burst activity of individual neurons, the wave was associated with inhibition of firing. Thomas, Schmidt, and Ward (18) also recently studied single cortical cells under the influence of strychnine. They find that the second phase of the diphasic impulse recorded with microelectrodes from single cells is increased by strychnine and that the first phase is unaffected. This contrasts with their finding no change in the shape of the unit potential recorded from single cells in experimental aluminum hydroxide epileptic foci.
Relation to Acetylcholine
It was inevitable in the present era of unitary hypotheses that attempts would be made to link the action of strychnine with acetylcholine. In 1938, Nachmansohn (19) showed that 1 in 30,000 strychnine sulphate solutions would produce 19 per cent inhibition of cholinesterase in vitro. However, strychnine is a considerably less potent anticholinesterase in vitro than physostigmine, neostigmine, or D.F.P. These latter drugs do not stimulate the convulsive actions of strychnine under any circumstances. It is true that there is a certain amount of synergism between strychnine and physostigmine (20), and between strychnine and acetylcholine (21). However, as we stated above, any mechanism that increases the amount of background activity exaggerates the effects of strychnine. The effect is seen with drugs with electrical and normal sensory stimulation, so that the synergism of strychnine with other anticholinesterases or with acetylcholine cannot be taken as a specific indication that strychnine is acting through its own weak anticholinesterase activity. All known anticholinesterases are antagonized by atropine, and strychnine can therefore be tested as an anticholinesterase by pitting it against atropine. The only support for strychnine as an anticholinesterase in vivo comes from the work of Longino and Preston (22). They used lethal doses of strychnine on mice and found that high doses of atropine did reduce the mortality rate. However, it may have been that at these high doses the peripheral effects of strychnine were predominant, and that atropine does affect this action. In contrast to this work, a very careful study by Wesco and Green (23) failed to find any effect of atropine on strychnine convulsions in cats with the rate and intensity of convulsions as the indicator. In support of this finding, Koppanyi (24) discovered that atropine, far from antagonizing the effects of strychnine, slightly decreased the threshold dosage required for the production of convulsions. It must be concluded that no satisfactory evidence has been produced that the in vivo convulsive activity of strychnine follows from its weak anticholinesterase properties. In peripheral structures, such as the peripheral ganglia of the crayfish, acetylcholine antagonizes the paralytic effects of strychnine (8) — a further point against the suggestion that strychnine acts via its anticholinesterase activity, similarly, Lanari and Luco (25) showed that prostigmine antagonized the paralytic action of strychnine on the cat neuromuscular junction and the superior cervical ganglion.
Relation of Strychnine to Other Drugs
The convulsive activity of strychninized tissue may be abolished by barbiturates. A quantitative study of the interaction of strychnine and barbiturates was carried out by Porter and Allamon (26). In strychnine neuronography, the spikes produced may be abolished by local application of nembutal. This technique allows the testing of more places than can be tested when it is necessary to await the dying-down of spiking before an application of strychnine in a new position. The interactions of narcotics and analeptics, especially strychnine and ephedrine, are described by Koll and Ergang (27) and by Ahlquist (28). The use of metrazol and strychnine for testing anticonvulsant drugs has been reported by Orloff, Williams and Pfeiffer (29). Even such a weak analeptic as quinine is shown to potentiate the effects of strychnine (30). It is of some interest in the location of strychnine activity that myanesin has been shown by Berger (31) and by Kaada (32) to be a powerful antagonist to strychnine convulsions. Calcium either injected systemically as gluconate or applied locally was found by Heinbecker and Bartley (33) to antagonize the convulsions. Since calcium increases accommodation and eliminates the prolongation of the period of latent addition, it was believed to be antagonizing through these two effects, as discussed in the next section. Ammonium chloride potentiates and enhances the drug (34).
Mode of Action of Strychnine on the Peripheral Nervous System
The general effect of strychnine on peripheral nervous structures, as reported by most authors, is depression with no signs of the generation of spontaneous activity. As might be expected these peripheral effects are particularly remarkable in the invertebrates. The first study after that of Luchsinger and Guillebeau (35) was the work of the Lapicques (36) on frog sciatic which showed a depression and block of nerves with a decrease of chronaxie. Differential change in chronaxie was used to explain the blocking action at the nerve muscle junction; this was later shown by Knoefel (37) not to be the case. Further, Bouman (38) showed that the chronaxie change did not appear if, instead of using the appearance of a minimal muscle response, the method of the half maximal response advised by Hill (39) was used. If, however, an excess of potassium was added to the strychnine, no effects could be seen under any form of stimulation. An observation of this kind stresses the fact that the effects of strychnine on peripheral structures have been determined under conditions greatly differing from those found necessary by later workers (40) to maintain the nerve in its normal state. It is therefore now most difficult to interpret the significance of these interesting results on peripheral structures. More detailed work on peripheral axons began with the work of Coppee and Peugnet (41, 42). It was greatly extended and, in general, confirmed by Heinbecker and Bartley (33). All concentrations of strychnine sulfate below 1 p.p.m. were found to decrease the height of single-action potentials. Coppee and Peugnet found an increase in the area of the action potential and a considerable prolongation, although this was not confirmed by Heinbecker and Bartley. In order of decreasing sensitivity to strychnine poisoning, B fibers were followed by A and C fibers. In very dilute solutions (less than 1:1,000,000) they occasionally observed a moderate lowering of the threshold of excised nerves, but at higher concentrations the threshold was always raised. At the same time strychnine inhibits the development of accommodation in the nerve, and the period of latent addition is increased — an effect which, as the authors point out, is similar to anodal polarization.
Finally, results of a quite different nature were found by Erlanger, Blair, and Shoepfle (43), who tested the variations of response latency and threshold at threshold stimulation of a peripheral nerve, the phalangeal nerve of the frog. Anodal or cathodal polarization was found not to affect the fluctuations, although cooling increased them. These fluctuations were quite random in their occurrence. It was found that low dilutions of strychnine which hardly raised the threshold produced a very great increase in the range of the oscillations; thus when the average threshold was increased by only 12 per cent, the oscillations increased by 394 per cent.
Studies on the Details of Strychnine Action in Some Locations
The action of strychnine on the spinal reflexes has been intensively studied in attempts to determine whether the effects represented an increase of excitation or a decrease of inhibition. Bradley and Eccles (44) recently reviewed and investigated this matter and concluded that strychnine decreases the direct inhibitory action of group la afferent impulses. If this were the main effect of strychnine, one would expect no effect on the size of the monosynaptic reflex, and this is the case, as reported by a number of authors. However, the action on the monosynaptic reflex was shown by Bernhard, Taverner, and Widen (45) to depend on the type of preparation used. They report that an increase of both mono- and poly-synaptic lumbar reflexes is seen only with low spinal sections. In decerebrate or high spinal animals the effect on the monosynaptic reflex is variable or absent, while the polysynaptic reflexes are greatly increased. It seems likely, therefore, that descending inhibitory systems can counterbalance the tendency of the monosynaptic reflexes to increase. The marked ability of the descending inhibitory systems from the cerebellum and the reticular system to abolish spinal tetanus was shown by Lettvin (46) and Terzuolo (12). It seems probable therefore that strychnine does not simply decrease all types of inhibition in spite of the evidence that direct inhibition is decreased.
The slow potential changes in the cord during strychninization have been recently reviewed by Brenner (4, 5) and by Brooks and Fuortes (47). In the frog and cat, the dorsal and ventral root potentials are increased in height and duration. Suddenly, these potentials break into a regular oscillation, about 4-10 per second in the frog and 10-30 in the cat. Bursts of impulses run out of the ventral roots during the negative phase of these oscillations. It is suggested that the combined action of strychnine and the arrival of afferent impulses depolarizes the motor horn cells. Bremer (5) was unable to find evidence that the spinal interneurons participated in the generation of the tetanus. Ajmone Marsan, Fuortes, and Morossero (48) show that an induced steady depolarization is sufficient to explain both the hyperexcitability and the tetanus. However, van Harreveld and Feigen (49) studied directly the polarization of elements in the ventral horn of the spinal cord and show that although barbiturates, for example, depolarize these elements, strychnine had no effect up to the time that convulsive activity started; at that time, as one would expect, a functional depolarization of the cells occurred. If this work is correct, it would be difficult to maintain that the initiation of strychnine effects on the ventral horn cells was attributable to their depolarization, since one would expect to see some effects before the beginning of convulsions.
It can well be imagined that if studies on the spinal cord, the kind queen of the nervous system, have resulted in conflicting answers to the nature of the action of strychnine, gross studies on the cortex have only added to the chaos. Studies such as those of Chang (50), and Bartley, O’Leary, and Bishop (51) have been useful in differentiating certain factors in cortical responses. In the absence of data on the exact origin of the potentials recorded, we can only conclude that while strychnine has a general excitatory effect it may exert either excitatory or inhibitory effect on certain components of the cortical response.
Experiments
In an attempt to resolve some of the questions posed by the preceding review, experiments of three types were carried out.
Methods
The special experimental conditions are described in each of the three sections.
Recording apparatus was standard: cathode follower head stages were coupled through Grass preamplifiers to a Dumont two-beam oscilloscope (No. 279).
Dorsal roots were stimulated in the usual way. Current pulses through the bipolar microelectrodes were generated by a special high impedance circuit, to insure constant current.
The microelectrodes consisted of two lengths of 10-micron platinum wire, separated by 5-10 microns of glass and coated with another 5-10 microns of glass to make a cylinder about 50 microns in diameter. Such cylinders were about 1 centimeter long, invariant in diameter, and sharpened at the tip to an eccentric fine point, one wire open at the very tip, the other some distance up the bevel. Where localization of the microelectrode was required, it was cut off and left in the spinal cord and determined histologically by the method described in the paper by Howland et al (52).
Experimental Results
1. The effect of strychnine on the threshold of ventral horn cells and terminal arborizations of afferent fibers.
Cats were anaesthetized with 0.5-0.7 cc per kilo of Dial injected intraperitoneally. The lumbar enlargement was exposed and placed under oil. Dorsal and ventral roots of the seventh lumbar segment were cut and prepared for recording. Intravenous curare was administered in order to control the expected strychnine convulsions. Artificial respiration and temperature control was maintained throughout the experiment. A bipolar microelectrode of the type described above was placed in the ventral horn of the seventh lumbar segment. Such an electrode was used in order to limit the main effects of the stimulation to the ventral horn. The preparation differs from that used by Renshaw (53) in that he was using a monopolar electrode and thus saw two volleys appear on the ventral root: the earlier one representing direct excitation of motoneurons, the later one arising from stimulation of afferents to motoneurons. We adjusted the stimulus strength so that only the direct volley for stimulation of the motor horn cells themselves was recorded on the ventral root. At that time, an antidromic volley was also recorded on the dorsal root of the same segment and represented the direct stimulation of the terminal arbor of those afferent fibers descending into the ventral horn. The ventral and dorsal root responses to two stimuli are shown at the top of Fig. 1. Two checks were made to confirm that the volleys recorded on the dorsal and ventral roots were not maximal and therefore could increase. First, the stimulus strength was reduced as low as was consistent with the appearance of a stable volley in the dorsal and ventral roots. Next, the animal was subjected to 90 seconds of asphyxia; the expected cycle of increase and decrease of the height of both volleys occurred, and was followed by a return to the resting state.
Next, 0.2 cc of a saturated solution of strychnine sulphate was injected intravenously. The result is seen in the middle pair of traces in Fig. 1. The height of the direct volley in the ventral root is unchanged but is now followed by an indirect volley. This second discharge is that reported by Renshaw (53) and shown by him to follow the stimulation of afferents to the ventral horn. Although there is no change in the size of the direct ventral root response, there is a small decrease in the area as well as in the height of the antidromic volley in the dorsal root. Recordings were taken just before generalized convulsions began. The barbiturate anesthesia had increased the threshold for these convulsions.
Although 0.2 cc of strychnine intravenously is enough to produce generalized fits in unanesthetized animals, the dose was next increased by a further injection of 0.3 cc of saturated strychnine sulphate solution. Again it is clear (lower pair of traces in Fig. 1) that there is no change in the height of the ventral root direct volley. A slight increase occurs in the height of the indirect volley following the second stimulus. A further decrease in the height of the dorsal root volley has occurred, 39 per cent below its original height. Similar results were obtained if a monopolar stimulating microelectrode was used, although in this case the great increase of the indirect response was much more obvious.
It can therefore be concluded that there is no change in the threshold of directly stimulated motor horn cells even with very large doses of strychnine. At the same time, there is an increase in the threshold of the terminal arborizations of the afferent fibers in the ventral horn.
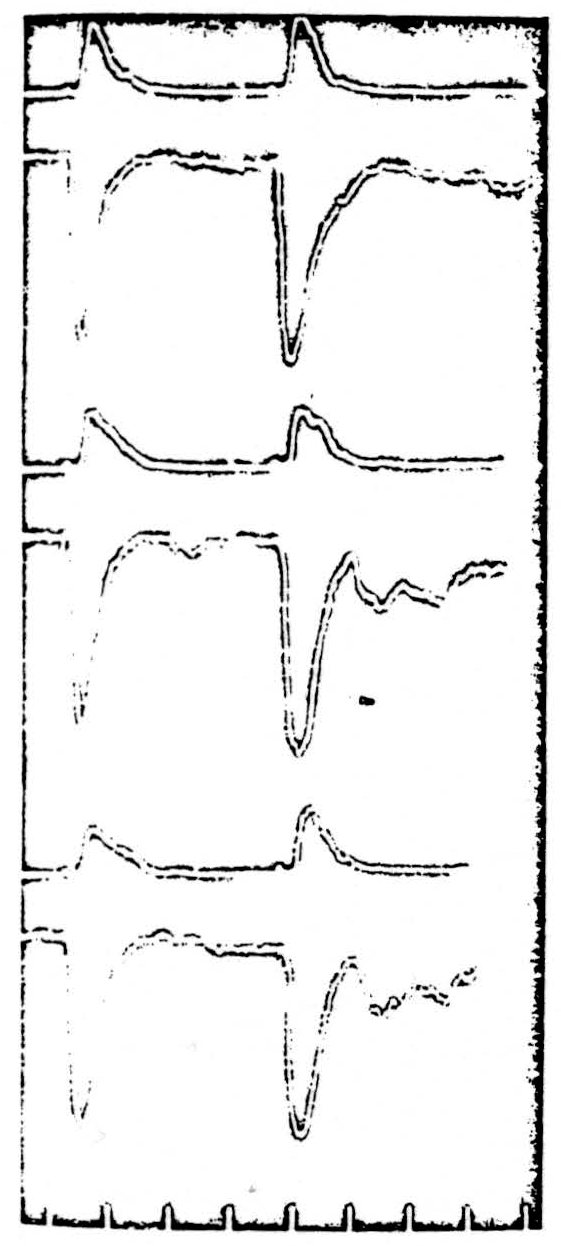
Figure 1. Each pair of traces shows (upper) the response traveling antidromically in the dorsal root and (lower) the response orthodromically in the ventral root. Two succeeding stimuli are given by a bipolar microclectrode in the ventral horn of the segment of the recording roots (L7). ** Upper pair: normal responses of the sensory afferent fibers and the motor horn cells.** ** Center pair: responses after 0.2 cc IV strychnine.** ** Lower pair: responses after a further 0.3 cc strychnine.** ** Bottom: time in milliseconds.**
There is no change in the height of the volley evoked from the motor born cells; there is a decrease in that from the terminal afferent arborizations.
The Effect of Strychnine on the Early Components of the Dorsal Root Potential
Five cats were used in this series of experiments. Three were anaesthetized with 0.5 cc per kilo intraperitoneal Dial; two were spinal preparations, initially anaesthetized with ether, the spinal cord sectioned at C1 after carotid and basilar artery occlusion. Similar results were obtained with both types of preparation. The lumbar enlargement was exposed and covered with oil kept saturated with 5 per cent CO2 and 95 per cent O2. Temperature was regulated throughout the experiment. The seventh lumbar dorsal root was cut in its canal and divided into two parts. One part was placed on stimulating electrodes 2 centimeters from the cord. The other part was prepared as for recording the dorsal root potential (DRP): one electrode close to but not touching the cord; the other on the crushed end of the nerve 2 centimeters away. The severed ventral root of L7 also lay on recording electrodes for monitoring. Curare and artificial respiration were used.
A supramaximal stimulus to one root produced the typical sequence of the dorsal root potential in the neighbor root. This complex consists of five waves, identified and analyzed by Lloyd and McIntyre (54), of which the fifth and largest negative wave is the dorsal root potential recorded by Barron and Mathews (55). The transition from the fourth to the fifth wave is marked by the appearance of the dorsal root reflex traveling antidromically in the dorsal root. Since we are concerned with the afferent fibers, only the first four components are shown. First, in Fig. 2A we show the normal shape of the dorsal root potential as a solid line. Next, the result of post-tetanic potentiation is shown as the dotted line in Fig. 2A. For 2 seconds, 100 supramaximal impulses per second were delivered to the stimulated root, then a single shock was delivered at the height of the potentiating effect of this tetanus. It will be seen that there is no effect on the first three waves, but that the fourth shows a decrease in its height and a prolongation.
Next, 0.2 cc of saturated strychnine sulphate solution was injected intravenously; the result is seen as the dotted line in Fig. 2B. It shows a prolongation of the fourth wave. Ten minutes later a second dose of 0.2 cc was injected, and in Fig. 2C further prolongation of wave 4 has occurred, now accompanied by a decrease in height. The second and third waves are now increased in height and delayed. This effect was further exaggerated when a third dose of 0.2 cc strychnine was injected 10 minutes later (Fig. 2D). The fifth wave was tremendously increased and the ventral root reflex was increased in both its mono-and polysynaptic components.
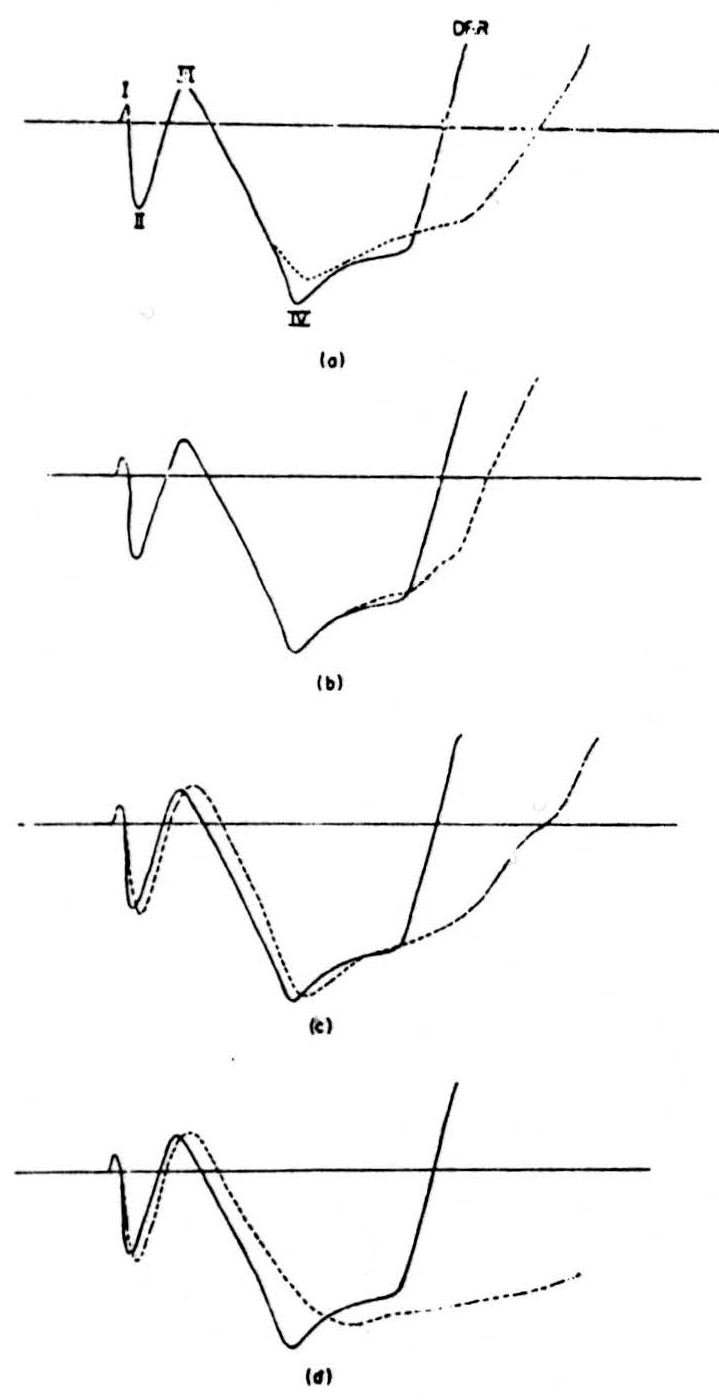
Figure 2. (a) Continuous line shows the shape of the first four components of the dorsal root potential as recorded in one preparation. The dotted line shows the effect on these potentials of a tetanus delivered to the recording root. (b), (c), (d) The continuous line shows the shape before strychnine of the first four compo-nents. The dotted line shows the effect of (b) 0.2 cc, (c) 0.4 cc, and (d) 0.6 cc of intravenous strychnine on the shape of these components.
Generation by Strychnine of Antidromic Volleys in the Dorsal Column
It has been known since the work of Gotch and Horsley in 1898 that strychnine convulsions in the spinal cord were accompanied by antidromic volleys in the dorsal roots. The occurrence of dorsal root reflexes in ordinary spinal preparations makes it difficult to interpret the significance of such volleys in the presence of strychnine. We therefore examined the other large ending point of primary afferent fibers, the nuclei cuneatus and gracilis. An antidromic volley in the medial lemniscus does not produce an antidromic volley in the dorsal columns in the absence of strychnine. Therefore, in order to see whether or not local application of strychnine in the nucleus gracilis would result in antidromic
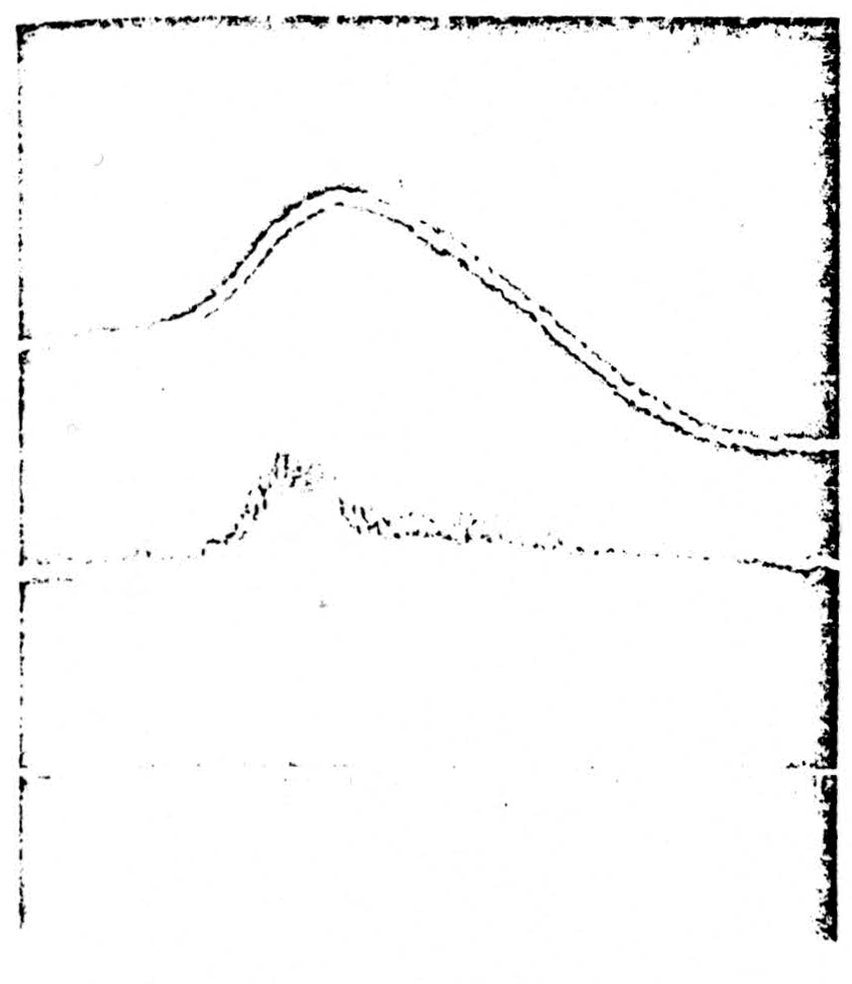
Figure 3.
Top: strychnine was applied to nuclei cuneatus and gracilis. The trace shows a single spike being generated within the nucleus. Center: at the same time a recording is taken from the seventh lumbar dorsal root 2 cm from the cord. An antidromically running spike is seen running out in the root. Bottom: time in 2 milliseconds and 10 milliseconds
“spike” conduction down the dorsal columns, cats were prepared under barbiturate anesthesia and the lumbar enlargement exposed in the manner described in the previous two sections. The caudal end of the fourth ventricle was exposed. Application of filter paper soaked in strychnine to the surface of the nucleus gracilis failed to produce strychnine spikes in several cases. It was therefore necessary to inject strychnine (one drop through a 26-gauge tube) directly into the nucleus. This was followed by the appearance of spiking activity in the region of the nucleus. Recordings were taken from the nucleus (Fig. 3, top) and from electrodes placed 2 cm from the cord on the cut L7 dorsal root (Fig. 3, center). It will be seen in Fig. 3 that the beginning of the generation of a “strychnine spike” in the nucleus is soon followed by the appearance of an antidromically conducted volley in a dorsal root. Section of the dorsal columns immediately abolished this transmission. It is concluded that in this case a strychnine spike was conducted antidromically from the nucleus down the dorsal columns and out of the sensory root.
Discussion
The results of the first set of experiments seem to show that strychnine does not affect the threshold of the motor horn cells. It is clear that if this is so, a number of the previous theories are untenable. We are supposing that the type of stimulus used fired off the dendrites or cell bodies of the motor horn cells rather than, or as well as, the axons. Evidence for this is: First, since both direct and indirect responses were seen, the stimulating current affected not only the most ventral regions where the axons of the motor horn cells are present but also fibers running to the motor neurons. Yet only the indirect responses were increased. Second, in other experiments we have found that an afferent volley does affect the threshold of the motor neurons tested in the way of the first set of experiments. Third, anodal make and cathodal make stimulation of the ventral horn cells interact in different ways with an incoming afferent volley. We presume that this indicates that the anodal and cathodal pulses were stimulating different parts of the motor cells. Strychnine failed to affect the threshold of the motor horn cells whether anodal or cathodal stimulation was used. Therefore, while it is true that we do not know what part of the cell is stimulated by a microelectrode in the ventral horn, the stimulated point can have its threshold affected by an afferent volley of nerve impulses. Yet the threshold of this point of stimulation is not affected by strychnine.
The increase of threshold of the primary afferent terminals and the changes in the shape of the early components of the dorsal root potentials both suggest an action of strychnine on activity in the primary afferent fibers. The results of Bradley and Eccles (44) could be taken to support this view. We have not yet measured the nature of this change, and it is questionable whether any satisfactory interpretation could be made of a dc shift recorded directly on a passive root. We suspect a hyperpolarization, at least of the intramedullary course of some of the fibers, since the increase of the first three waves of the dorsal root complex suggests axons in the so-called nerve reaction state. This suspicion is further heightened by the measurable increase in threshold of the afferent fibers intramedullarly. Furthermore, the changes being in the same direction as that found after post-tetanic potentiation suggests a similar process both with regard to the sign of potential shift in the membrane and the increased effectiveness of signals traversing such fibers. It is therefore now of particular interest to know the effect of strychnine on normal peripheral nerve fibers, although it is possible that the terminal arborizations of fibers might react differently.
The results from the third set of experiments may have significance beyond the simple demonstration that antidromically traveling strychnine spikes may be generated. It is possible, of course, that there are some descending fibers in the dorsal columns; such tracts have been demonstrated in species other than the cat. However, none of these descending tracts sends fibers out of the dorsal roots as far as is known. Since the recordings shown in Fig. 3 were taken from the dorsal root, there is little doubt that these were, in fact, antidromically running impulses in sensory fibers. A second possibility that has to be ruled out is that the strychnine applied to the nuclei of Goll and Burdach had activated some system in the medulla which projected into the spinal cord and there generated the equivalent of a dorsal roof reflex so that the impulses recorded in the dorsal root were not those that had descended dorsal column sensory fibers. The ragged start of the spike in the nuclei and in the root does not allow us to exclude this possibility by an accurate measurement of conduction time. However, we have purposely stimulated descending systems, including the whole spinal cord with dorsal columns cut, and have been unable to elicit any antidromic volley out of the dorsal roots. Therefore it seems likely that our results indicate a true generation of synchronized volleys in the dorsal column terminal arborization. These impulses might play a part in the synchronization of the firing of the cells in the nucleus.
Now that we have evidence for antidromic impulses, we naturally ask whether they originated in the terminal arbor or were transmitted across the synapse antidromically. The latter does not seem to be the explanation. Stimulation of the medial lemniscus did not result in the appearance of a volley in the dorsal columns even under strychnine. Similarly, antidromic stimulation of the ventral roots in a strychninized cord does not result in antidromic impulses running out of the dorsal roots. It therefore seems most likely that these impulses originated in the terminal arborization of the dorsal column fibers. Such generation of impulses of greater effectiveness than normal might explain the synchronized detonation of all the cells supplied by a particular fiber, as well as increased ephaptic interaction with other afferents. While this may not be a complete explanation for “strychnine spikes” it does account for some of the characteristics.
Critique of Strychnine Neuronography
The prolonged and careful development of the technique by Dusser de Barenne, McCulloch, and others is described in a number of papers (2, 3, 13). It must be emphasized that at no time did they believe that this method supplanted those beautiful but increasingly rare anatomical tracings of pathways upon which our definite knowledge of neuro-anatomy is based. It was intended only to provide a general scheme of organization of fibers, indicating those areas in which histological methods might be most profitable. We may restate and examine the postulates on which the usefulness of the technique is based.
Local application of strychnine results in a synchronous discharge of cells within the region. We examined, under general effects of strychnine, the five locations in which it has been reported that strychnine does not have this effect. It seems reasonable to point out that these locations contain somewhat unusual cells; three are in the autonomic system, one in the cerebellum, and one in the olfactory bulb. Further, it is possible that within a region giving off strychnine spikes some cells may be inhibited by the discharge of others, as suggested by the work of Li (17). It is therefore necessary to modify the general statement with the warning that certain types of cell within a region may not contribute to the spike-producing activity. This caveat is not as crippling as suggested by Chow and Hutt (56) or by Frankenhaeuser (10). In the instances in which Dusser de Barenne and McCulloch used negative findings as evidence for an absence of direct connections, spikes occurred where the strychnine was placed; this was not the case in Frankenhaeuser’s or Wall’s and Horwitz’s work.
Strychnine applied to axons at some distance from cell bodies does not generate spiking activity. The work we present here suggests that strychnine may lead to the generation of activity in axons close to their termination or cells. However, there is no doubt that strychnine applied along the main nonbranching course of an axon does not evoke activity.
Spikes travel orthodromically. The authors of this paper show one clear example of antidromic transmission of a strychnine spike. In defense of the results of strychnine neuronography on the cortex, the responses recorded at some distance from the strychninized areas were not volleys traveling in tracts but were the arrival of synchronized bursts of impulses in the region of cells giving rise to large transients, much slower than a nerve spike.
An ink-writer is not equipped to handle signals as short as nerve spike or an antidromic volley to cortex, especially since the slow potential accompanying the latter is very easily abolished by barbiturates, the drug of choice in neuronography. Thus, while antidromic impulses from strychnine can occur, only high frequency recordings will show them. The work done on old instruments with low frequency response probably does not contain evidence of these antidromic spikes.
Strychnine spikes do not cross synapses. Synapses rarely transmit impulses in such a way that output repeats input. It was shown in many locations that the arrival of a synchronized burst of impulses, the strychnine spike, was not followed by the response of the next set of cells with a similar degree of synchronization. The only exception was found by Wall and Horwitz (11) in the stellate ganglion where the unusual one-to-one impulse transmission occurs. Thus this postulate may still be applied in most known cell groups.
It is therefore evident that considerable care is needed in the interpretation of data derived from strychnine neuronography. The technique, however, remains valid as a method for the rapid survey of probable connections.
Summary
- Some of the literature on the basic effects of strychnine is reviewed.
- Experiments carried out on cats show that strychnine does not affect the excitability of the motor horn cells of the spinal cord as tested by microelectrode stimulation within the cord.
- It is shown that the threshold of the fibers in the terminal arborization of final afferent fibers is raised. The shape of the early components of the dorsal root potentials is changed. Findings could be explained by an increase in the height of the individual action potentials in the terminal arbors under the influence of strychnine.
- Strychnine spikes traveling antidromically away from strychninized nuclei of Goll and Burdach were shown to emerge from lumbar dorsal roots.
- The significance of these findings is discussed. A critique of strychine neuronography in the light of recent findings is presented.
Footnotes
Bibliography
Magendie, F., Some examination of the action of some vegetables on the spinal marrow. Institute of France, April 24,1809.
Dusser de Barenne, J.G., The Mode and Site of Action of Strychnine in the Nervous System, Physiol., Rev., 1933, 13, 325-335.
Dusser de Barenne, J.G., Marshall, C., Nims, L.F., and Stone, W.E., The Response of the Cerebral Cortex to Local Application of Strychnine, Am. J. Physiol., 1941, 132, 776-780.
Bremer, F., Le Tetanos, Strychnique el le Mechanisme de la Synchronisation Neuronique, Arch, int. Physiol., 1941, 51, 211-260.
Bremer, F., Strychnine Tetanus of the Spinal Cord, Ciba symposium on the spinal cord, Little, Brown and Co., Boston, 1953, 78-83.
Munch, J.G., Gorlough. F.E., and Ware, J.C., Bioassays of Rodenticides, J. Amer. Pharm. Asoc. 1936, 25, 744-746.
Viehoever, A., and Cohen., L, Mechanism of the Action of Strychnine, Am. J. Pharm., 1937, 109, 285-316.
Bonnet, V., Action of Strychnine and Acetylcholine on Neuronic Rhythmicity in Crustaceans, Compt. rend. Soc. de biol., 1938, 127, 804-806.
Dow, R.S., The Electrical Activity of the Cerebellum, J. Physiol., 1938, 94, 67-86.
Frankenhaeuser, B., Limitations of the Method of Strychnine Neuronography, J, Neurophysiol., 1951, 14, 73-79.
Wall, P.D., and Horwitz, N., Observations on the Physiological Action of Strychnine, I. Neurophysiol., 1951, 14, 257-263.
Terzuolo. C., Supraspinal Influences on Spinal Strychnine Tetanus, Arch. Int. Physiol., 1954, 62, 179-196.
Dusser de Barenne, J.G., and McCulloch, W.S., Functional Organization of the Sensory Cortex of the Monkey, J. Neurophysiol., 1938, 1, 69-85.
Busquet, H., and Vischniac, C, Action of Strychnine and Brucine on the Spinal Cord, Compt. rend. Soc. de biol., 1938, 128, 729-732.
Adrian, E.D., and Moruzzi, G., Impulses in Pyramidal Tract, J. Physiol., 1939, 97, 153-199.
Jalvisto, E., Reflex Motor Discharge in Single Fibers of the Frog in Strychnine Poisoning, Acta physiol. scand., 1945, 9, 313-335.
Li, C.L., Functional Properties of Conical Neurons with Special Reference to Strychninization, American Electroencephalographic Soc.,. 9th ann. meeting, 1955.
Thomas, L.B., Schmidt, R., and Ward, A. A., Observations on Single Units in Chronic Cortical Epileptic Foci and in Normal or Strychninized Cortex, Amer. E.E.G. Soc. 9th ann. meeting 1955.
Nachmansohn, D., Mechanism of the action of strychnine on the nervous system, Compt. Rend. Soc. de Biol., 1938, 129, 941-943.
Hamed, B.K., and Cole, V.V., Synergism of Physostigmine and Strychnine, Proc. Soc. Exper. Biol, and Med., 1938, 39, 372-376.
Oti, Y., Effect of Ach or Epinephrine on Spinal Reflex Action of Strychnine, Okayama-Igakkai-Zasshi, 1940, 52, 25-77.
Langino, F.S., and Preston, R.S., Antagonism between Atropine and Strychnine, J. Pharmacol, and Exper. Therap., 1946, 86,174-176.
Wesco, W.C, and Green, R.E., Lack of Atropine Antagonism to Strychnine, J. Pharmac. and Exp. Therap., 1948, 97, 78-84.
Koppanyi, T., The Action of Toxic Doses of Atropine on the Central Nervous System, Proc. Soc. Exper. Biol, and Med., 1939, 40, 244-248.
Lanari, A., and Luco, J.V., Depressant Action of Strychnine on Superior Cervical Sympathetic and on Muscle, Am. J. Physiol., 1939, 126, 277-282.
Porter, E.L., Allamon, E.L., Quantitative Study of Barbiturate-Strychnine Antagonism, J. Pharmacol, and Exper. Ther., 1936, 58, 178-191.
Koll, W., and Ergang, M., Antagonistic Action of Narcotics and Analeptics, Arch, f. exper. Path, u. Pharmakol., 1942, 199, 577-605.
Ahlquist, R.P., The Synergism of C.N.S. Stimulants, J. Amer. Pharm. Assoc., 1947, 35, 414-415.
Orloff, M.J., Williams, H.L., and Pfeiffer, C.C., Timed Intravenous Infusion of Strychnine or Metrazol for Testing Anticonvulsant Drugs, Proc. Soc. Exp. Biol. and Med., 1949, 70, 254-257.
Gessner, O., Potentiating Effects of Strychnine by Quinine, Arch, f. Exper. Path. u. Pharmakol., 1942, 202, 363-365.
Berger, F.M., The Mode of Action of Myanesin, Brit. J. Pharm., 1947, 2, 241-250.
Kaada, B.R., Action of Myanesin, J. Neurophysiol., 1950, 13 ,89-104.
Heinbecker, P., and Bartley, S.H., Mode of Action of Strychnine on the Nervous System, Am. J. Physiol., 1939, 125, 172-187.
Ajmone Marsan, C., Fuortes, M.G.F., and Marosserro, F., Influence of Ammonium Chloride on the Electrical Activity of the Brain and Spinal Cord, E.E.G. Clin. Neurophysiol., 1949, 1, 291-298.
Luchsinger, B., & Guillebeau, A., Fortgesetzte Studien zu einer algemeinen Physiologie der irritabeln Substanzen, Arch, f. d. ges. Physiol., 1882, 28, 1-60.
Lapicque, L. and M., Action de la Strychnine sur I'Exitabilite du Nerf Moteur, Compt. Rend. de la Soc. de Biol., 1907, 62, 1062-1064.
Knoeel, P.K., Strychnine and Chronaxie, Am. J. Physiol., 1936, 638-641.
Bourman, H.D., Experiments on the Mechanism of Strychnine Curarization, J. physiol., 1936, 88, 328-340.
Hill, A V., The Strength-duration Relation for Electric Excitation of Nerve, Proc. Roy. Soc. B. 1936, 119, 440-443.
Lorente de Nó, R., A Study of Nerve Physiology, Rockefeller Inst. Med. Res., 1947.
Coppee, G., and Peugnet, H.B., Action of Strychnine on Peripheral Nerves, Compt. verd. Soc. de biol., 1936, 123, 283-286.
Coppee, G., and Coppee-Bolly, M.H., Action of Strychnine on Isolated Nerve, Arch, int. de physiol., 1941, 51, 97-129.
Erlanger, J., Blair, E.A, and Schoepfle, G.M., Spontaneous Oscillations in Excitability of Nerve Fibers, Am. J. Physiol., 1941, 134, 705-718.
Bradley, L, and Eccles, J.C., Strychnine as a Depressant of Primary Inhibition, Nature, 1952, 171, 1061-1062.
Bernhard, C.G., Taverner, D., and Widen, L., Differences in the Action of Tubocurarine and Strychnine on the Spinal Reflex, Brit. J. Pharm., 1951, 6, 551-559.
Lettvin, J.Y., The Path of Suppression in the Spinal Grey Matter, Fed. Proc. No. 1, Part I, March 1948, 71.
Brooks, C.M., and Fuortes, M.G.F., Potential Changes in the Spinal Cord following Administration of Strychnine, J. Neurophysiol., 1952, 15, 257-267.
Ajmone Marsan, C., Fuortes, M.G.F., and Marossero, F., Effects of Direct Current on the Electrical Activity of the Spinal Cord, J. Physiol., 1951, 113, 315-321.
van Harreveld, A., and Feigen, G.A., Effect of Some Drugs on the Polarization of Spinal Cord, Am. J. Physiol., 1950, 160, 451-461.
Chang, H.T., Strychnine on Local Cortical Potentials, J. Neurophysiol., 1951, 14, 23-28.
Bartley, S.H., O'Leary, J., and Bishop, G.H., Differentiation by Strychnine of Visual Integrating Mechanisms of the Rabbit Optic Cortex, Am. J. Physiol., 1937, 120, 604-618.
Howland, B., Lettvin, J.Y., McCulloch, W.S., Pitts, W., and Wall, P.D., Reflex Inhibition by Dorsal Root Interaction, J. Neurophysiol., 1955, 18, 1-17.
Renshaw, B., Activity in the Simplest Spinal Reflex Pathways, J. Neurophys., 3, 1940, 373-387.
Lloyd, D.P.C., and McIntyre, A.K., On the Origins of Dorsal Root Potentials, J. Gen. Physiol., 1949, 32, 409-443.
Barron, D.B., Mathews, B.H.C., Potential Changes in the Spinal Cord, J. Physiol., 1938, 92, 276-321.
Chow, K.L., and Hutt, P.J., The “Association Cortex” Macaca Mulatta: A Review of Recent Contributions to Its Anatomy and Junctions, Brain, 1953, 76, 625-677.
Dun, F.T., Restoration of the Dorsal Root Potential after Strychnine, Proc. Soc. Exper. Biol., 1942, 49, 479-480.
For further research:
Wordcloud: Action, Activity, Afferent, Antidromic, Cells, Change, Convulsions, Cord, Cortex, Decrease, Dorsal, Doses, Effect, Fibers, Followed, Found, Generated, Height, Horn, Impulses, Increase, Local, Motor, Nerve, Peripheral, Physiol, Potential, Produce, Recorded, Reflex, Response, Results, Root, Shown, Shows, Soc, Spikes, Spinal, Stimulation, Strychnine, Study, System, Therefore, Threshold, Used, Ventral, Volley, Work
Keywords: Strychnine, Fibers, Wiring, Reference, Self, Neuronography, Brain, Vegetables, Contributors
Google Books: http://asclinks.live/0bhf
Google Scholar: http://asclinks.live/utww
Jstor: http://asclinks.live/tt2a