AN ELECTRICAL HYPOTHESIS OF CENTRAL INHIBITION AND FACILITATION12 [99]
W.S. McCulloch, J.Y. Lettvin, W.H. Pitts and P.C. Dell
Introduction
No one doubts that synaptic transmission can be affected by chemical substances. As physiologists we often use them to alter it in one way or another. Particular substances may play an adjuvant rôle in this transmission but, for the sake of simplicity, we believe we should not look to these unknowns to explain things that can be understood in terms of electrical events. Therefore, we have proposed to account for synaptic transmission and its facilitation and inhibition in terms of the known electrical properties of neurons acting in the geometrical arrangements of the spinal cord. Though similar mechanisms presumably operate elsewhere in the nervous system, our present study is limited to the two-neuron arc anti factors which increase or decrease its response. The necessary experiments we have performed on spinal and decerebrate cats after all traces of anesthesia were gone, or on peripheral nerve under conditions as nearly as possible resembling those found in the body. In some cases we merely confirmed what others have found. In others we have succeeded in eliminating a number of ambiguities of interpretation arising from activity induced in subsidiary structures. In general, we believe we have refined the quantitative determination of effects sufficiently to ascertain whether or not these conformed to theory.
Our hypothesis fundamentally rests on potential theory and the well- known properties of neurons. Current flowing out of a cell excites it locally and, if strong enough, will initiate an impulse which may be propagated to all parts of the cell; whereas current flowing into a cell raises its threshold locally, and if sufficiently strong will prevent an impulse from traversing that region. For the anatomy of the parts we depend chiefly on Ramón y Cajal (1); having little independent data of our own, our anatomical knowledge is not yet as detailed as we desire.
Various parts of our physiological hypothesis have been held by others (2, 3, 4, 5, 6), but we know of no one who has combined them in our fashion. Figure 36 is Ramón y Cajal's picture of a typical motoneuron. We believe its dendrites extend even more widely so that when axons come from the dorsal root to it they must thread their way among its dendrites to end on its cell body. Figure 37 is Ramón y Cajal's picture of this approach of collaterals of dorsal root fibers.
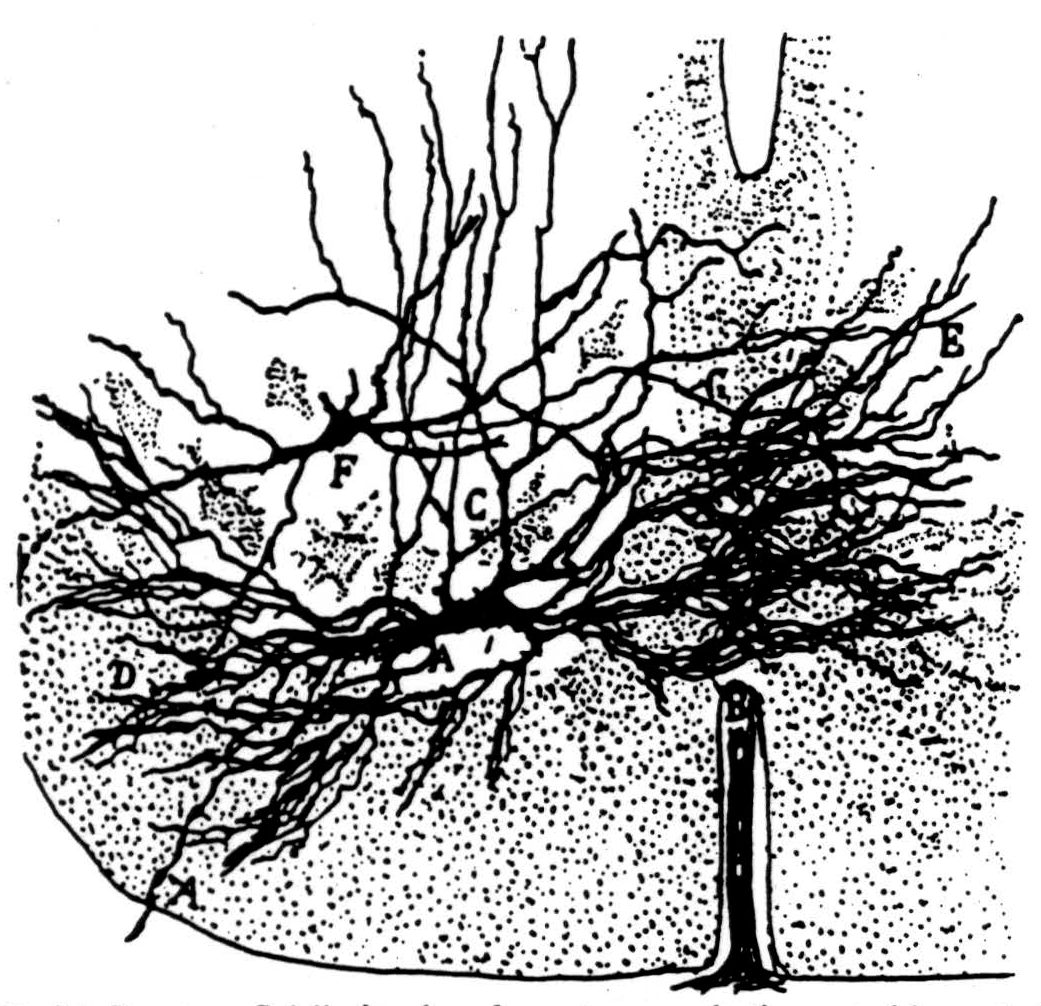
Figure 36. Ramón y Cajal's drawing of a motoneuron in the ventral born of the spinal cord. (From Ramón y Cajal: Histologic du système nerveux de l'homme et des vertébrés. Vol. I, Paris, A. Maloine, 1909.)
When an impulse comes to the end of a presynaptic arbor it dies there slowly, leaving a sink in the terminals upon the body and a source in the presynaptic arbor among the dendrites. When enough impulses arrive close to the cell body nearly simultaneously to excite it they initiate an impulse.
A volley from a muscle nerve insufficient to fire the cell sets up a distribution of currents in the presynaptic elements which a microelectrode tip placed very close to the cell records as a pure negative potential swing (7), called by us the presynaptic potential.3 Its electrotonic image appears in the ventral root. Provided the volley is insufficient to cause electrical activity of other structures, the presynaptic potential and its electrotonic image are of the same sign, shape and temporal course, namely, a rapidly

Figure 37. The sensorimotor collaterals (B) are shown emerging from the dorsal white matter and traveling through the region of the intermediate nucleus to end on the motoneurons. (From Ramón y Cajal: Histologie du système nerveux de l'homme ct des vertébrés, Vol. I, A. Maloine, Paris, 1909.)
developing negativity which fades slowly. In Figure 38 three pairs of curves obtained with increasing afferent volleys are presented to show that the electrotonic potential is linearly related to the presynaptic potential. This is to be expected if the motoneuron merely acts as a leaky core-conductor. When the afferent volley is of yet greater strength the response of the motoneuron is recorded from the immediate vicinity of the cell body
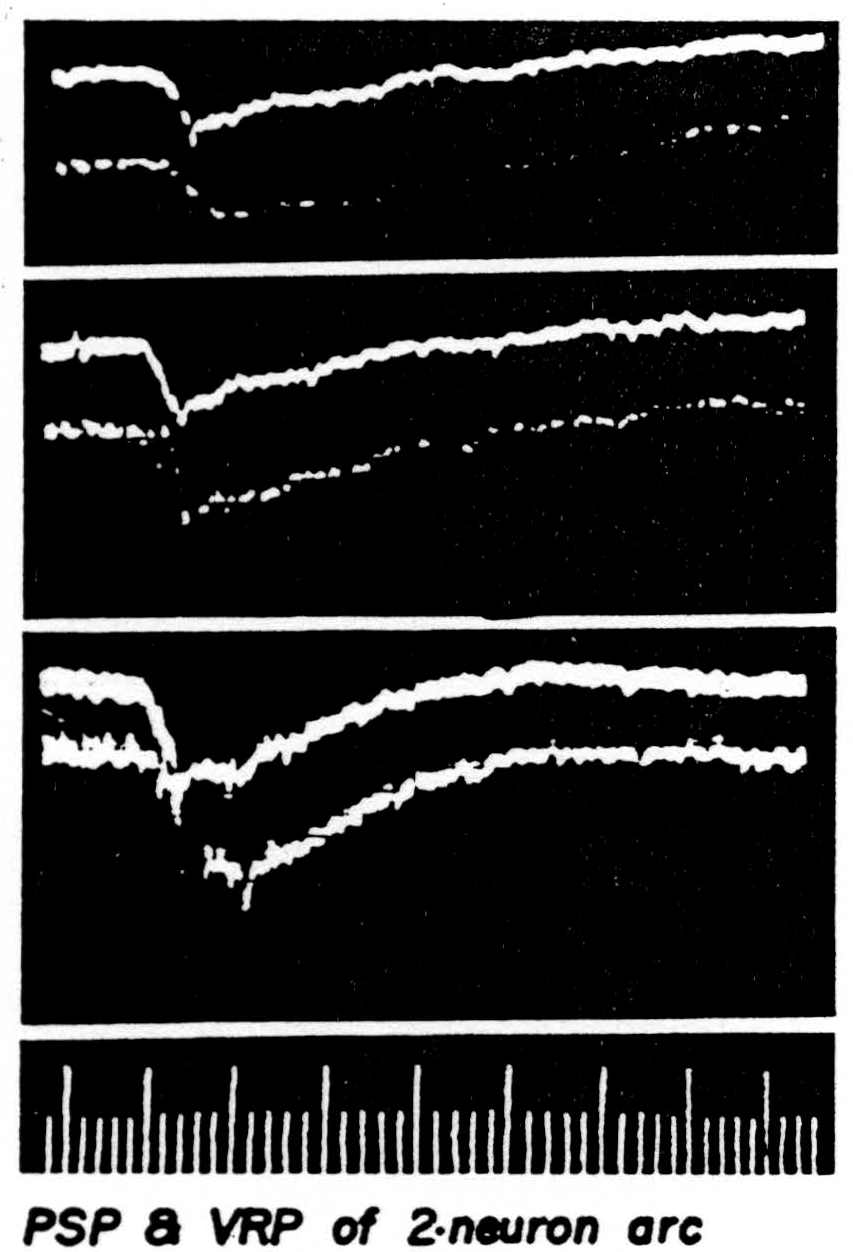
Figure 38. The presynaptic potential is the upper curve of each picture. Its image in the ventral root slightly less than 1 mm. from root exit is the lower curve. The presynaptic potential was recorded slightly away from a single unit so that no spike would appear. The cat had been decerebrated, then spinalized between L1 and L2 under ether and all dorsal roots caudal to the section severed save L7 and S1 on one side. The medial and lateral gastrocnemius nerves of that side were stimulated as a unit. Negative is recorded down in this set of pictures. The electronic switch oscillates at 50,000 per sec. Time: 1 and 5 msec.
as a negative spike, while at a little greater distance among the dendrites the spike is positive. Still farther the spike vanishes. These findings conform to the proposed origin of the presynaptic potential and of the spike.
In the bulbo-reticular formation near the midline in the medulla (8) there are large cells whose axons descend in the ventromedial funiculus, whence they emerge to excite adjacent internuncials (9) (see Fig. 39). According to Cajal, the fine axons of these internuncials ascend or descend several segments in the ventral funiculus whence fine branches emerge to ramify among the motoneurons ascending and perhaps ending upon their dendrites (see Fig. 40).
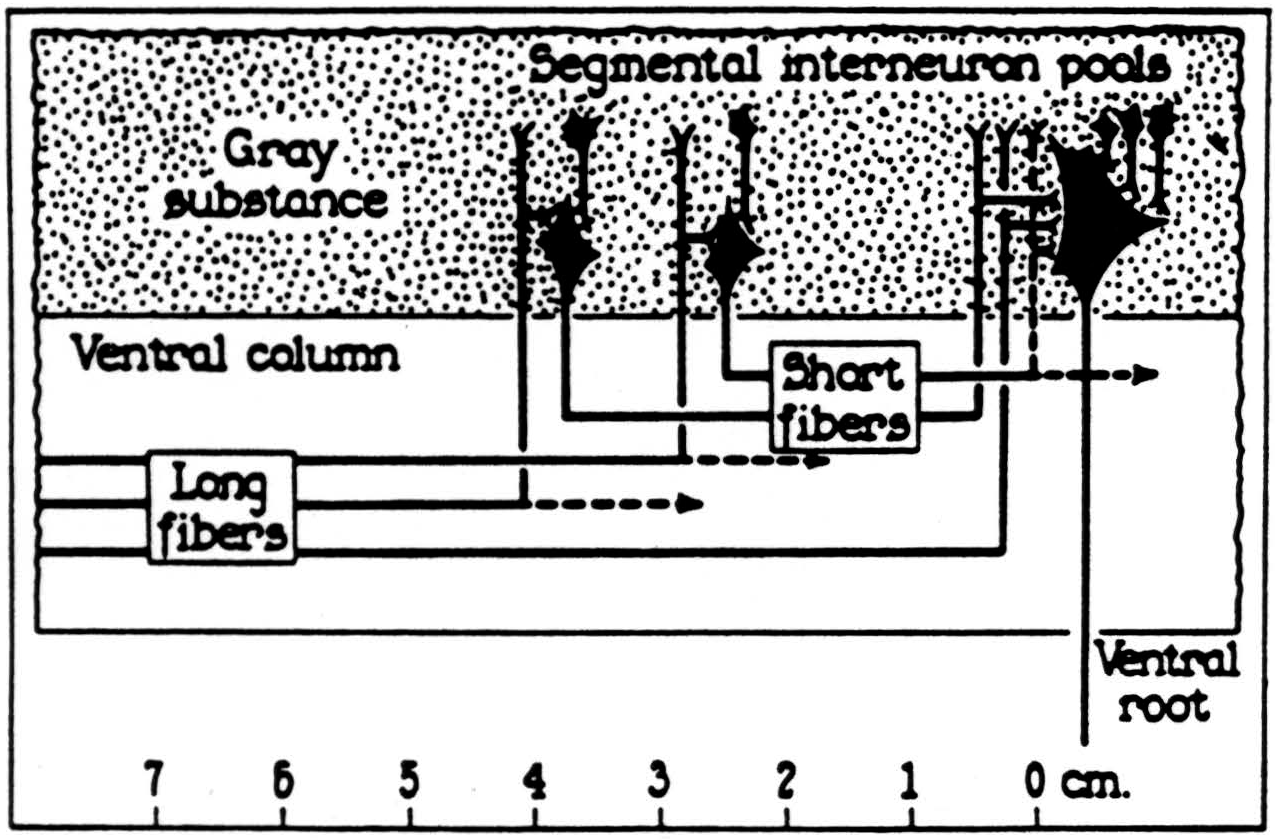
Figure 39. Lloyd's diagram of the bulbo-spinal correlative system. The ventral magnocellular elements in the reticular system of the medulla send long axons down the ventromedial funiculus which end on intemeurons at the ventromedial aspect of the ventral bom. These send short fibers up and down the ventral funiculus which give off collaterals to the ventral horn over several segments. (From Lloyd: J. Neurophysiol., 4: 115-134, 1941.)
Impulses from these internuncials dying among the dendrites make their diffuse sinks there and their sources near the bodies of the motoneurons. Hence they raise the threshold of the motoneurons, as we can infer from the diminished response to an antidromic volley.4
Accompanying the polarization of the motoneurons there is a positive electrotonic potential in the ventral root (5), while the dorsal root fibers, which are polarized simultaneously, exhibit a negative electrotonic image of it on the dorsal root (Fig. 41). Both are evoked by a single pulse applied to the bulbar-reticular formation at the beginning of the sweep. Since it is
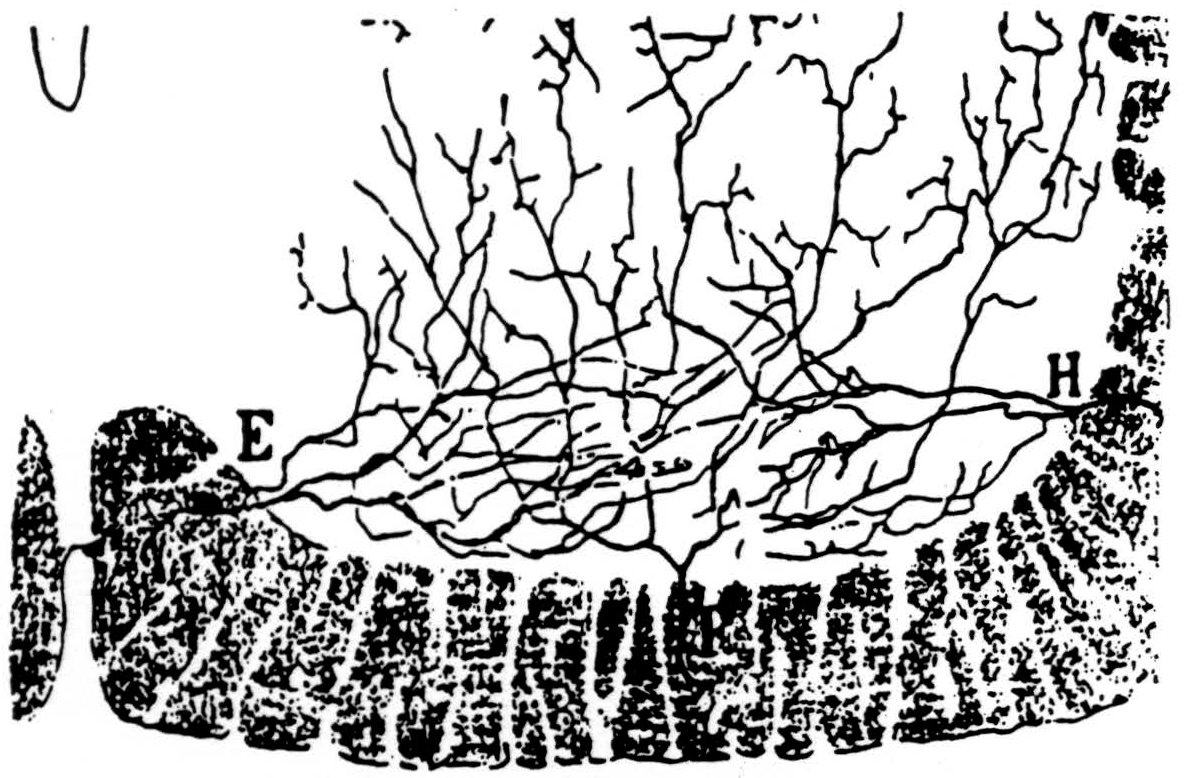
Figure 40. Ramón y Cajal's drawing of collaterals entering the ventral born from the ventral funiculus. Some of these are explicitly said by him to arborise along tbe dendrites. (From Ramón y Cajal: Histologie du Système nerveux de l'homme et des vertébrés Vol. I, A. Maloine, Paris, 1909.)
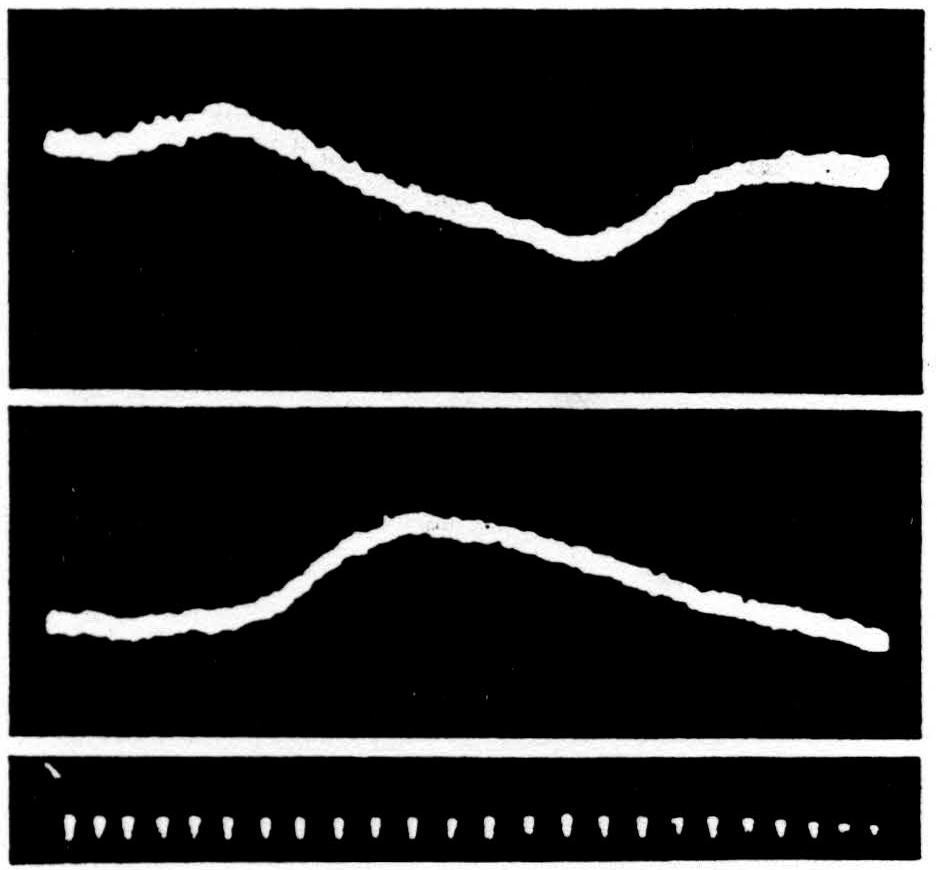
Figure 41. Above is the ventral root potential, below the dorsal root potential at S1 following application of a single pulse to the bulbo-reticular inhibitory formation (the ventral magnocellular cell group of the bulbar-reticular formation) given at the beginning of the sweep. Inhibition of spinal reflexes occurs during the period of these potential changes. Negative is recorded upwards. Time indicated in intervals of 5 msec.
the large terminal portions of the sensorimotor collaterals that are driven positive, impulses coming by way of the dorsal root will be blocked some distance from the cell.
Hence during bulbo-reticular inhibition of the two-neuron arc the presynaptic potential near the cell body will be markedly diminished and the negative spike of the cell will be absent. Figure 42 shows such an inhibition
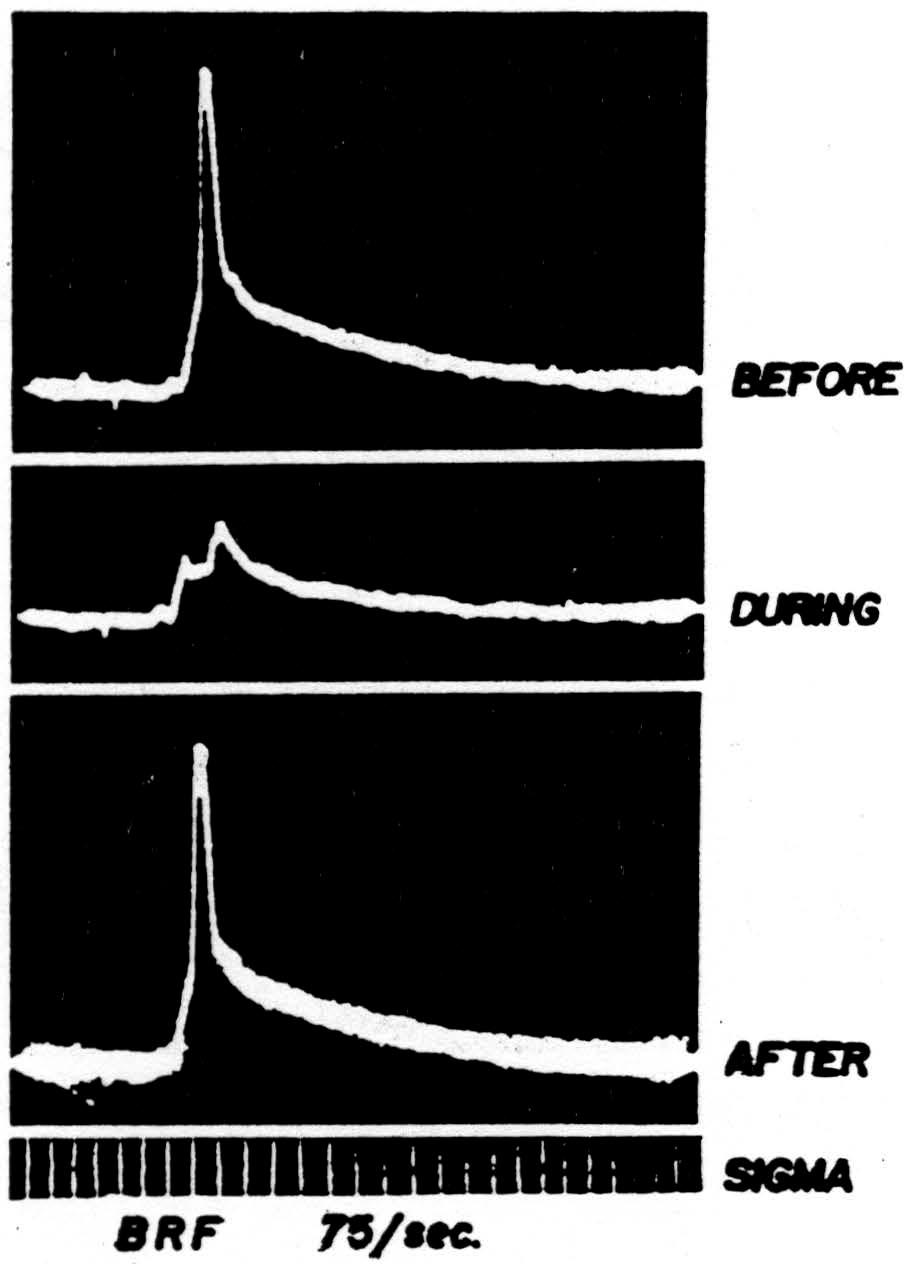
Figure 42. The response of a single motoneuron and the presynaptic potential in the gastrocnemius nucleus recorded by a microelectrode before, during, and after bulbo-reticular stimulation at 75 per sec. Time: 1 msec. Low frequency filters used.
(second record) with antecedent and subsequent controls. It was taken during repetitive stimulation of the bulbo-reticular formation at 75 per sec. As in most systems involving many internuncials, the effect increases with repetition.
Thus there are two ways of producing inhibition postsynaptic inhibition, which acts by increasing the threshold of the portion of a cell to be ex-nerve in the saline it will be affected by the same flow of current as an electrode at that place—i.e., in-out-in, in that order—at any place at succeeding times and at any one time at successive places. Thus the conditioning nerve will affect the test nerve in the same way that any other applied electromotive force would do so—that is, it will inhibit, facilitate and inhibit it in that order. This interaction, first suggested by Adrian, has been demonstrated experimentally by Marrazzi and Lorente de Nó (10) and by Rosenblueth and confirmed by us as part of the following experiment.
Regardless of how a nerve is blocked, an impulse approaching the block is altered in much the same way, that is, the spike goes as far as it can, and dies there. As it dies its height diminishes at all points without moving along the nerve. Lorente de Nó has plotted this very precisely.
Figure 43 shows how we have found the voltage of a bullfrog nerve to die away at a series of points two millimeters apart along our conditioning nerve C. To obtain the rate of change of the slope at each place we recorded the difference in voltage between an electrode at that place and the average value of two others one millimeter on each side of it. Two examples of this are shown by the dotted lines in the inserts on the right. At each point along the nerve the excitability of the test nerve was measured by stimulating it there with a constant submaximal stimulus and recording the size of the spike at the other end. This was done at various times during the arrival and decay of the conditioning volley. The dashed inserts at the right show how the volley in the test nerve was facilitated or inhibited at points 4 and 8 in the diagram below, and allow direct comparison of this effect with the rate of change of slope at these points (dotted lines).
From these facts it is apparent that as we approach the region of block the volley in the conditioning nerve, which well to the left yielded inhibition, facilitation, inhibition (10), yields now only inhibition, followed by a facilitation which lasts as long as the sum of the facilitation and second inhibition of the triphasic curve. But at the region of block and beyond we find inhibition and only inhibition, reaching its maximum when the spike has gone as far as it can and dying slowly thereafter with a time constant about four-tenths of a millisecond in these myelinated axons. Two of these series of measurements are shown in Figure 44 as recorded.
The success or failure of an impulse in traversing a region of decrement produced by local pressure is very sensitive to any currents in that region. It is therefore a sensitive method of detecting facilitation and inhibition in the test nerve produced by an impulse dying at a block in a conditioning nerve. The test nerve may be stimulated at one end and the response recorded at the other while the point of pressure in the test nerve is moved along the conditioning nerve. The results are essentially the same as in the previous experiment.
We shall make use of these findings in interpreting the interaction of presynaptic elements in the spinal cord (6) where, we assume, the rise in internal resistance which occurs in fine fibers chiefly at branch points constitutes a hazard to conduction because of the sudden reduction in diameter of the fine branches.
Ramón y Cajal described two kinds of terminal aborizations of axons, the one relatively thick, dense and bunched, the other fine, sparse and splayed. In the bunched arbor an impulse in any one branch will tend to facilitate a contemporaneous impulse in a neighboring branch where that impulse


Figure 44. Recordings of the envelope of interaction at points comparable to 4 and 8 of figure 43 but from a different experiment.
might otherwise die; but in a sparse arbor the impulse in one branch is of little assistance to that in any other, so that the tendency will be great for impulses to die short of the anatomical termination of the fiber. Imagine now, coming toward a single cell body—both approaching the body from among its dendrites—two mingled arbors, one sparse, the other dense. If impulses come by both of them simultaneously their effect will differ but little from that of the dense arbor alone, whereas when the impulse in the sparse precedes the one in the dense and dies short of the cell body it will stop the impulse in the dense at the place where its own impulse has died. Because of its very sparseness it will generate little potential in the vicinity of the body of the recipient cell and practically no accompanying electrotonic image in the emergent axons. That is what happens. If we place a microelectrode somewhere near the cell body and try the combined effect of the inhibitory volley and a test volley we shall not even expect much diminution of the presynaptic potential. We usually find none. At such a point we often record the spike of that cell as positive, for we are nearer the dendrites than the cell body. But when the microelectrode is practically on the cell body, so that we record its spike as negative, inhibition may diminish the presynaptic potential somewhat.5
Finally, consider what will happen if we bury one dense arbor in another. Each will tend to facilitate the other maximally when they conduct simultaneously, and decreasingly thereafter at a decreasing rate which will be the same as the decreasing rate at which inhibition falls off. This, in essence, is what Lloyd has found to be the temporal course of monosynaptic facilitation and monosynaptic inhibition in the spinal cord (11).
Footnotes
References
Ramón y Cajal, S.: Histologie du sysème nerveux de l'homme et des vertébrés. Vol. I, Paris, A. Maloine, 1909.
Brooks, C. McC. and Eccles, J. C.: An electrical hypothesis of central inhibition. Nature, 159: 760-764, 1947.
Brooks, C. McC. and Eccles, J. C.: Inhibitory action on a motor nucleus and focal potentials generated therein. J. Neurophysiol., 11: 401-418, 1948.
Brooks, C. McC. and Eccles, J. C.: Inhibition of antidromic responses of motoneuroncs. J. Neurophysiol., 11: 431-444, 1948.
Brooks, C. McC., Eccles, J. C. and Malcolm, J. L.: Synaptic potentials of inhibited motoneurones. J. Neurophysiol., 11: 417-430, 1948.
Renshaw, B.: Observations on interaction of nerve impulses in the gray matter and on the nature of central inhibition. Am J. Physiol., 146: 443-448, 1948.
Brooks, C. McC. and Eccles, J. C.: Electrical investigation of the monosynaptic path way through the spinal cord. J. Neurophysiol., 10: 251-474, 1947.
Magoun, H. W.: Bulbar inhibition and facilitation of motor activity. Science, 100: 549-550, 1944.
Lloyd, D. P. C.: Activity in neurons of the bulbospinal correlation system. J. Neurophysiol., 4: 115-134, 1941.
Marrazzi, A. S. and Lorente de Nó, R.: Interaction of neighboring fibers in myelinated nerve. J. Neurophysiol., 7: 83-101, 1944.
Lloyd, D. P. C.: Facilitation and inhibition of spinal motoneurons. J. Neurophysiol., 9: 421-438, 1946.
Eccles, J. C.: Synaptic potentials of motoneurones. J. Neurophysiol., 9: 87-120, 1946.
Lorente de Nó, R.: A study of nerve physiology. Studies from the Rockefeller Institute for Medical Research, 131 and 132: 1947.
Lorente de Nó, R. and Laporte, Y.: Synaptic transmission in a sympathetic ganglion. J. cell. comp. Physiol., 35: Supplement 2, 9-192, 1950.
For further research:
Wordcloud: Arbor, Axons, Block, Body, Branch, Brooks, Bulbo-Reticular, Cajal, Cell, Collaterals, Conditioning, Dendrites, Dense, Dies, Dorsal, Eccles, Effect, Electrical, Electrotonic, End, Facilitation, Fibers, Figure, Formation, Impulse, Inhibition, Interaction, Lorente, Motoneurons, Negative, Nerve, Neurophysiol, Points, Potential, Presynaptic, Ramon, Recorded, Region, Response, Root, Spike, Spinal, Synaptic, System, Test, Ventral, Volley
Keywords: Inhibition, Transmission, Cell, Nerve, Experiments, Substances, Theory, System, Cond
Google Books: http://asclinks.live/pzz5
Google Scholar: http://asclinks.live/rgy2
Jstor: http://asclinks.live/wxoc