RAPID CHANGES IN CEREBRAL OXYGEN TENSION INDUCED BY ALTERING THE OXYGENATION AND CIRCULATION OF THE BLOOD [69]
E. Roseman, C.W. Goodwin and W.S. McCulloch
Introduction
Studies conducted in the Laboratory of Neurophysiology at Yale had shown large and rapid changes in the pH (1, 2) and lactic acid (3, 4) content of the cerebral cortex. These were such as to indicate the possibility of equally large and rapid concomitant fluctuations in the oxygen tension. Such changes have now been followed by the simplified polarographic method described below. In this article are presented enough of the results to indicate that the electrode system does follow rapid fluctuations in the oxygen tension of the brain produced by alteration of the oxygenation and circulation of the blood.
The conditions studied were respiratory anoxia produced by breathing pure nitrogen, increased cerebral blood flow secondary to a rise in systemic blood pressure after the injection of adrenalin, and reduced cerebral blood flow secondary to decreased systemic blood pressure resulting from generalized vasodilation and to generalized skeletal muscle paralysis.
Method
All experiments were performed upon cats of about 2.5 kg., anaesthetized with nembutal, 30 mg. per kilogram, given intraperitoneally. A tracheal cannula was installed. The scalp was incised and four electrodes, two on each side, were implanted in the skull. A trephine hole 10 mm. in diameter was made about 6 mm. from the midline, and the dura mater was incised to permit implantation of the oxygen electrodes1. These consisted of an outer glass tube, just under 10 mm. external diameter, containing a stiff saline agar jelly. The positive Ag-AgCl electrode was buried in the saline agar. Through the center of the agar ran a second glass tube surrounding the negative platinum electrode, except for the tip which was bare platinum wire no. 28, extending about 1.5 mm. beyond the rest of the electrode. When implanted, the platinum wire pierced the entire thickness of the cortex to enter the white matter, while the agar jelly rested on the surface of cortex and dura mater. A little plasticine was then worked into the crack between the skull and the tube, and the junction was painted with collodion repeatedly, to insure rigidity and to make the connection air tight.
In the standard polarographic procedure, current through such an electrode system is plotted as a function of the voltage across the system. There appear plateaus corresponding to the concentration of various substances in the solution. In particular, the plateau between 0.55 to 0.7 volts is well known to depend upon a reaction involving oxygen. The height of this plateau—i.e., the amperage, varies with the O2 tension. The center of the plateau remains in the vicinity of 0.62 volts.
In the early experiments the current was measured by a galvanometer. When this proved to be too slow, 0.9 volts was applied through 1 megohm resistor, across which the current produced a proportional voltage. This was input into a Goodwin direct-coupled differential amplifier (5) and thence into a DuMont electronic switch, whose output was led to a Grass power amplifier and associated ink-writer. The envelope of the resulting trace served to measure the oxygen tension (6). Two other channels of the same instrument were used to record the electroencephalogram (E. E. G.) and another to record the electrocardiogram (E. K. G.).
Under a moderate nembutal anesthesia, with the head at the level of the heart so as to insure good circulation of the brain, the normal frequency of spontaneous respiration of cats was about 30 per minute, and there was a relatively constant current flowing through the O2 tension electrodes. Artificial respiration was then initiated from a Starling Ideal respirator pump delivering 50 c.c. per stroke, at 30 strokes per minute, which served to maintain the current through the O2 tension electrodes very near its original value. No cat attempted to breathe against this artificial respiration. Therefore, these parameters of artificial respiration were used as standard conditions in subsequent procedures. In order to avoid movements, animals were usually injected with a drug having a curare like action. Of these, Dihydro Beta Erythroidine Hydrobromide2 was found most reliable for the required procedures and was used almost exclusively.
When the constant value of the current was established, the intake of the pump was connected to a carefully balanced Spirometer (circa 80 liters) containing O2 and the current was observed until it had reached a maximum value. Then the intake was again opened to room air until the current had re-equilibrated. This procedure was then repeated with the spirometer filled with Ns until all signs of cortical activity had disappeared, when room air was admitted. Finally artificial respiration was turned off until cortical activity had disappeared, when room air was again admitted. Drugs affecting the circulation were next injected. At the end of the experiment the effect of O2, N2; and cessation of artificial respiration were tested again.
Results
Since the induced changes in cortical oxygen tension were observed most frequently in cats paralyzed with erythroidine, its effects are presented first in Fig. 1. This shows the fall in heart rate and subsequent partial recovery, each accompanied by a corresponding change in cortical oxygen tension, which is here expressed in arbitrary units. The sign of these units represents the direction of deviation from the resting O2 tension of the cortex under the standardized conditions. Comparison with Fig. 2 indicates that the fall in O2 tension (6 to 7 units) is a little less than one-third of the fall induced by respiration of N2. These curves, for initial total doses of 1 to 2 milligrams of erythroidine, are extremely reproducible and subsequent doses have a similar but smaller effect. Paralysis is complete and lasts typically about one hour. Neither the frequency nor the amplitude of the electrical activity of the cortex is represented in Fig. 1, for there was no appreciable change in frequency and only a slight, transient diminution in amplitude.
The only available curare had no visible effects on any of these observables but produced only a momentary paralysis. Intocostrin in the large doses sufficient to paralyze respiration, produced no visible alteration, but the paralysis was too fleeting for present purposes.
In order to avoid complicating results gratuitously, no subsequent procedure was begun until half an hour after intravenous injection of erythroidine. By that time the heart rate had returned to nearly its original level and O2 tension had reached the level which it maintained thereafter.
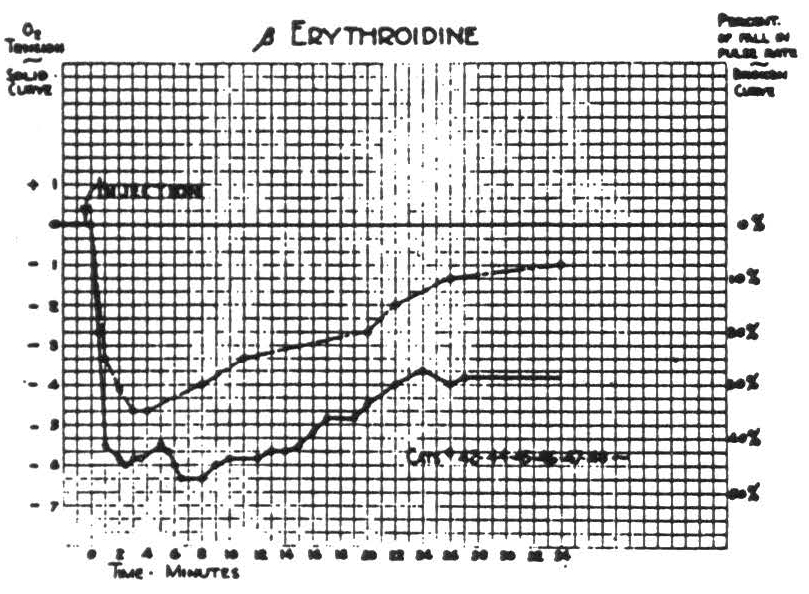
Figure 1.
Respiration of nitrogen causes an immediate sharp fall in the cortical O2 tension which at the end of a minute usually reaches its nadir, and at this time the electrical activity of the cortex has usually disappeared. It is of interest to note that when this activity has disappeared the O2 tension has already reached its lowest point. If air is not readministered the animal dies in another 1.5 to 2 minutes. If air is administered, the O2 tension rises almost as fast as it falls. This is accompanied by the return of the electrical activity. Figure 2 is a composite graph of this effect, based on repeated trials on the first eight animals. Experience with subsequent animals makes it seem unlikely that there is any significant difference between these curves of the paralyzed and nonparalyzed preparations.
Respiration of O2 causes a prompt rapid rise in O2 tension of cortex within the first half minute, followed during the second half minute by a slower rise to the final value which is maintained indefinitely. Readministration of room air causes it to fall rapidly but at a decreasing rate to a value usually slightly above its original level. Figure 3 is a composite graph based the amplitude in either direction and in the time of fall, resulting in a notching of the plateau not found in the individual records.
Nonparalyzed preparations struggle when air is occluded, and occlusion
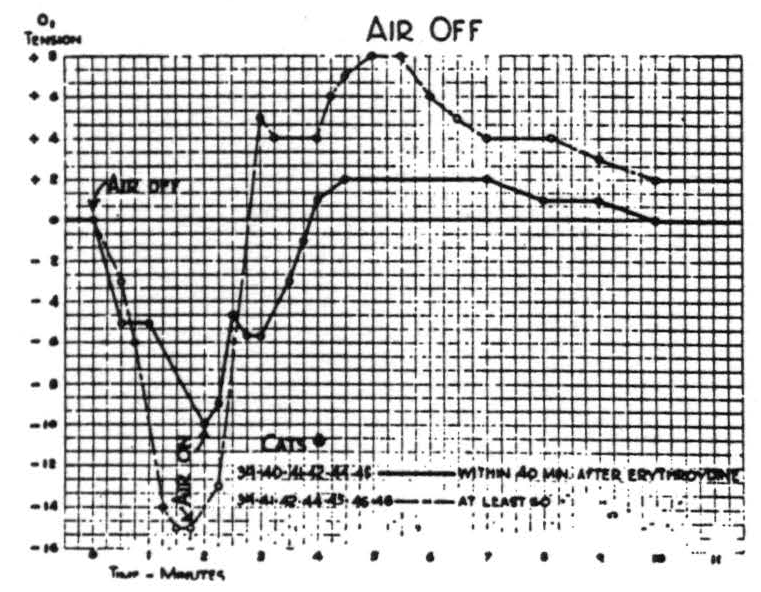
Figure 4.
leaves the oxygen in the preparation, which due to struggling will be more rapidly converted into CO2 and so effect circulation more profoundly. The effects of circulatory changes were therefore investigated by use of several drugs—namely, adrenalin, ephedrine, ergotamine tartrate, nicotinic acid
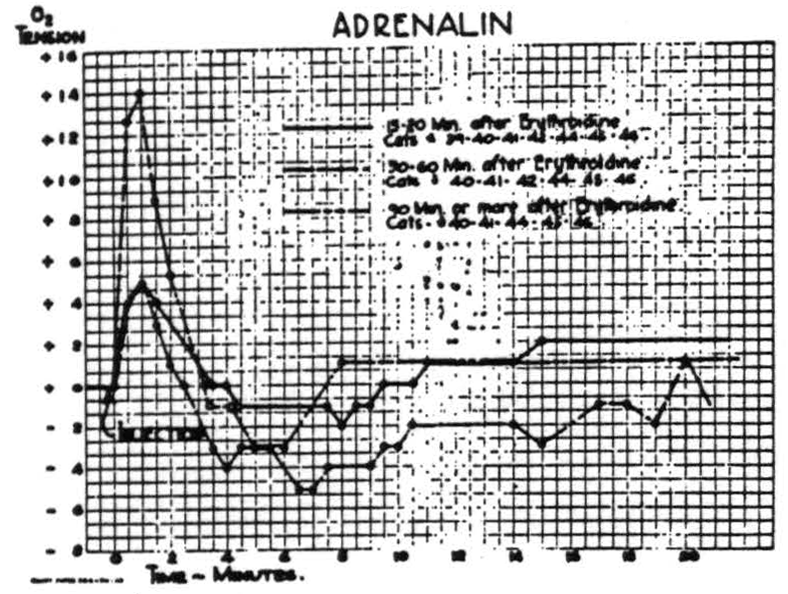
Figure 5.
and amyl nitrite. Of these, adrenalin was most extensively investigated by the intravenous injection of very large doses at various intervals after erythroidine. The results were reproducible and the graphs of Fig. 5 are average curves based on many injections in seven animals. While these curves differ in form and amplitude, due to the action of erythroidine, all indicate the dependence of the cortical O2 tension upon the systemic circulation. In comparable doses, ephedrine sulphate has a smaller and much on repeated procedures on the first five animals on which it was tried. Subsequent experience confirms the amplitude and the general form of the curve, as well as the lack of any significant difference between paralyzed and non-paralyzed
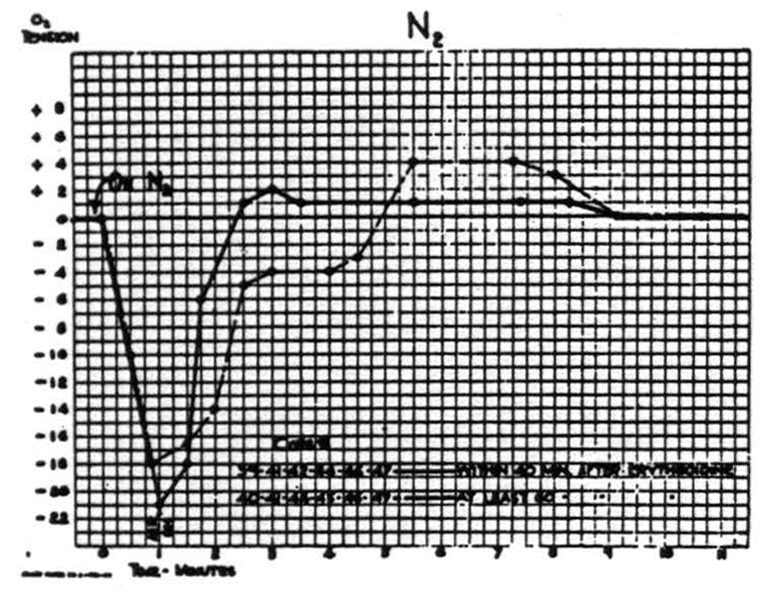
Figure 2.
preparations. No change in heart rate or electrical activity of the cortex was demonstrable.
On the other hand, paralysis does significantly alter the effects of occlusion of respiration. In both paralyzed and nonparalyzed preparations
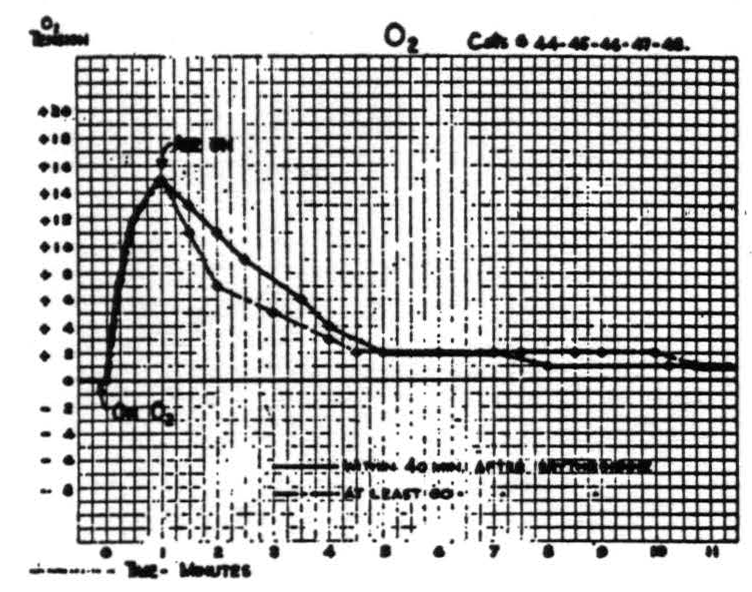
Figure 3.
the oxygen tension diminishes, though more slowly than with inhalation of nitrogen, and electrical activity disappears at a higher level of oxygen tension; but upon return of respiration there is an overshoot in O₂ tension. The difference is this: that in the nonparalyzed preparation all of the changes are significantly larger than in the paralyzed. Figure 4 is a composite curve indicating the average changes in the first eight animals on which the procedure was attempted. There is considerable individual variation, both in more prolonged effect upon cortical Os tension. Even 1 milligram of ergotamine tartrate intravenously has a barely perceptible but persistent effect. Nicotinic acid, which is said to increase cerebral blood flow, produced only slight and variable increases in oxygen tension. This was true even in doses of 50 to 100 milligrams except in one cat which showed a rise of 16 scale units sustained for half an hour. Amyl nitrite caused a sharp fall in O2 tension, and when given to animals who had a critically low O2 tension or when given in large doses, lowered the O2 tension to the lethal level.
Thus all of these drugs have shown the dependence of the oxygen tension of the cortex upon the systemic circulation.
Discussion
The attempt to measure polarographically the oxygen tension of tissues in vivo raises several questions that require consideration. It is well known that the current obtained with a given electrode system at a given oxygen tension, changes only slightly with changes in temperature of the entire system, and in these experiments no significant changes in temperature occurred. On the other hand, even the concentric arrangement of electrodes here employed is sensitive to thermal gradients within the system. To minimize such changes the experiments were performed in an air conditioned room whose thermal fluctuations were small and slow, and all other sources of thermal disturbances were kept at a distance.
Polarographic determination of any substance in solution depends upon the existence in the current voltage curve of a plateau corresponding to a reaction in the solution in the immediate vicinity of the noble metal electrode, and so upon the local concentration of the substance that receives the electrons. In the present experiments that substance is oxygen in solution. In ordinary unstirred solutions the consumption of oxygen at the electrode lowers its local concentration far below that of the rest of the solution; in fact, with a given electrode system, the current in adequately stirred solutions is many times as great as in unstirred solutions. In brain in vivo a second factor enters—namely, the oxygen consumption by brain, which, for an equal volume, is some fifty times as great as that of the platinum wire electrode. Of course this electrode does not have any capillaries supplying oxygen, whereas the comparable volume of brain does. Still, since both electrode and brain consume oxygen and both have to rely upon diffusion to supply it, and the rate of consumption by the electrode is so far less than that by brain, it seems only reasonable to expect that the current passed at a given oxygen tension will be nearer to that in a stirred than to that in an unstirred solution. This was confirmed by comparing the current passed through brain with the current which flowed when the same electrodes were immersed in stirred and unstirred solutions equilibrated with room air, for the O2 tension of brain, if calculated from the unstirred as a standard, was many atmospheres, whereas if calculated from the stirred it was a reasonable value, less than 30 mm. of Hg.
The absolute value of the current depends also upon the area of the platinum electrode and the condition of its surface, so that in order to interpret the current passed by a given electrode it is necessary to calibrate that electrode. The foregoing considerations indicate that there is no ready and sure method of so doing. The authors have therefore confined their interpretation to the relative changes from the initial value, comparing these in all cases to the maximum changes that could be induced by respiration of pure O2 and N2.
In order to minimize the effects of vertical displacements of the cortex along the platinum wire, it was made long enough to pierce the entire thickness of the cortex. What part of the current is referable to any layer of the cortex is immaterial to the present investigation, which was designed to disclose the rapidity of fluctuation in the relative oxygen tension of the cortex as a whole. Gradients of oxygen tension presumably exist about all vessels, and the use of a long electrode does not preclude the possibility that the absolute value of the current might have been appreciably different had the electrode been located a short distance laterally from its actual position—but again, this is immaterial for present purposes. Actually, the resulting currents with a given electrode were surprisingly consistent not only in any one animal but from animal to animal.
One other possible source of difficulty with the method must be mentioned. It is known that changes in the pH in the direction of acidity, tend to narrow the O2 plateau in the current voltage curve, the effect being more marked on the high voltage edge. However, the changes of pH of the cortex are not great enough for this to become a serious source of inaccuracy, except when extreme acidity is associated with extremely low values of O2 tension, and here the effect is to decrease the fall in current, making the O2 tension appear a little higher than it is. It is doubtful whether this played any significant role in any observations reported here.
Finally, the series resistor used was sufficiently large to produce a considerable voltage drop, with electrodes of large area. Due allowance was made for this by the application of 0.9 volts, so that under standardized conditions the voltage across the electrodes was approximately that of the center of the plateau due to oxygen. But the changes in current due to the induced changes in oxygen tension were sufficiently great to cause changes in the voltage drop of the series resistor which are unquestionably significant. Their effect is always such as to decrease alteration in current. Thus, except for very small changes, the record always indicates a change disproportionately smaller than that which actually occurs.
Of the findings, one is worthy of note—namely, that the O2 tension of brain can be elevated circa 75 per cent by respiration of O2 instead of air. As the majority of the oxygen is transported in combination with hemoglobin which is usually nearly all oxygenated in arterial blood, it is interesting that the increased oxygen tension of the blood which corresponds to a small increment in the concentration of O2 in the blood should have so great an effect on the O2 tension of brain. That this increased cortical O2 tension does not appreciably affect the cortical electrical activity suggests that it does not significantly alter the oxygen consumption by the cortex. This would mean that any additional oxygen would remain to keep up the cortical oxygen tension. Even so, to account for so great an increase in cortical oxygen tension on the basis of so small an increment in total oxygen supply in the blood stream, one must remember that this excess oxygen, being under about five times the usual partial pressure of oxygen in the blood stream, can be delivered at a much greater rate during the passage of the blood through the cortex.
Summary
With a platinum electrode, salt bridge and suitably adjusted voltage it is possible to record the oxygen tension of tissue in vivo as a function of the amperage. By this method studies were carried out on the cortices of cats anaesthetized with Dial and immobilized with Beta Erythroidine. A decrease in cortical oxygen tension invariably followed erythroidinization; this was believed to be due to a decrease in cerebral blood flow secondary to a decrease in systemic blood pressure. When adrenalin or ephedrine were administered, an increase in cerebral oxygen tension occurred; this was believed to be due to an increase in cerebral blood flow secondary to a rise in systemic blood pressure. Amyl nitrite caused a fall in oxygen tension probably secondary to the fall in systemic blood pressure. Ergotamine tartrate and nicotinic acid had insignificant effects. Inhalation of nitrogen produced a precipitous fall in cortical oxygen tension to a low level which was considered asphyxial. With the readmission of air, the cerebral oxygen tension rose rapidly to a level above that observed before asphyxiation. Breathing O2 raised the cerebral oxygen tension.
Footnotes
References
Marshall, C. S., McCulloch, W. S., and Nims, L. F. Observations on the pH of the arterial blood, the pH and electrical activity of the cerebral cortex. Amer. J. Physiol., 1938, 124: 631–636.
Marshall, C. S., McCulloch, W. S., and Nims, L. F. pH of the cerebral cortex and arterial blood under insulin. Amer. J. Physiol., 1939, 125: 680–682.
Stone, W. E. Some observations on pH, lactic acid and phosphates of the cortex. Amer. J. Physiol., 1940, 129: 475–476.
Stone, W. E., Marshall, C., and Nims, L. F. Chemical changes in the brain produced by injury and by anoxia. Amer. J. Physiol., 1941, 132: 770–715.
Goodwin, C. W. A differential amplifier for resting and action potentials of biological systems. Yale J. Biol. Med., 1941, 14: 101.
Davis, E. W., McCulloch, W. S., and Roseman, E. Rapid changes in the O, tension of cerebral cortex during induced convulsions. Amer. J. Psychiat., 1944, 100: 825–829.
For further research:
Wordcloud: Acid, Activity, Air, Animals, Blood, Brain, Cats, Cerebral, Changes, Circulation, Concentration, Cortex, Cortical, Current, Curve, Decrease, Doses, Due, Effect, Electrical, Electrode, Erythroidine, Experiments, Fall, Figure, Flow, Given, Increase, Indicate, Large, Level, Method, Observed, Oxygen, Ph, Plateau, Platinum, Present, Procedure, Produced, Rapid, Respiration, Results, Solution, Substance, System, Tension, Value, Voltage
Keywords: Studies, Tension, Changes, System, Blood, Secondary, Pressure, Cortex, Brain, Acid
Google Books: http://asclinks.live/ma8l
Google Scholar: http://asclinks.live/8h5r
Jstor: http://asclinks.live/9m3v