CHANGES OF NOBLE-METAL ELECTRODE EMF OF CEREBRAL CORTEX1 [66]
W.S. McCulloch, J.R. Klein and C. Goodwin (Introduced by R.W. Gerard)
Introduction
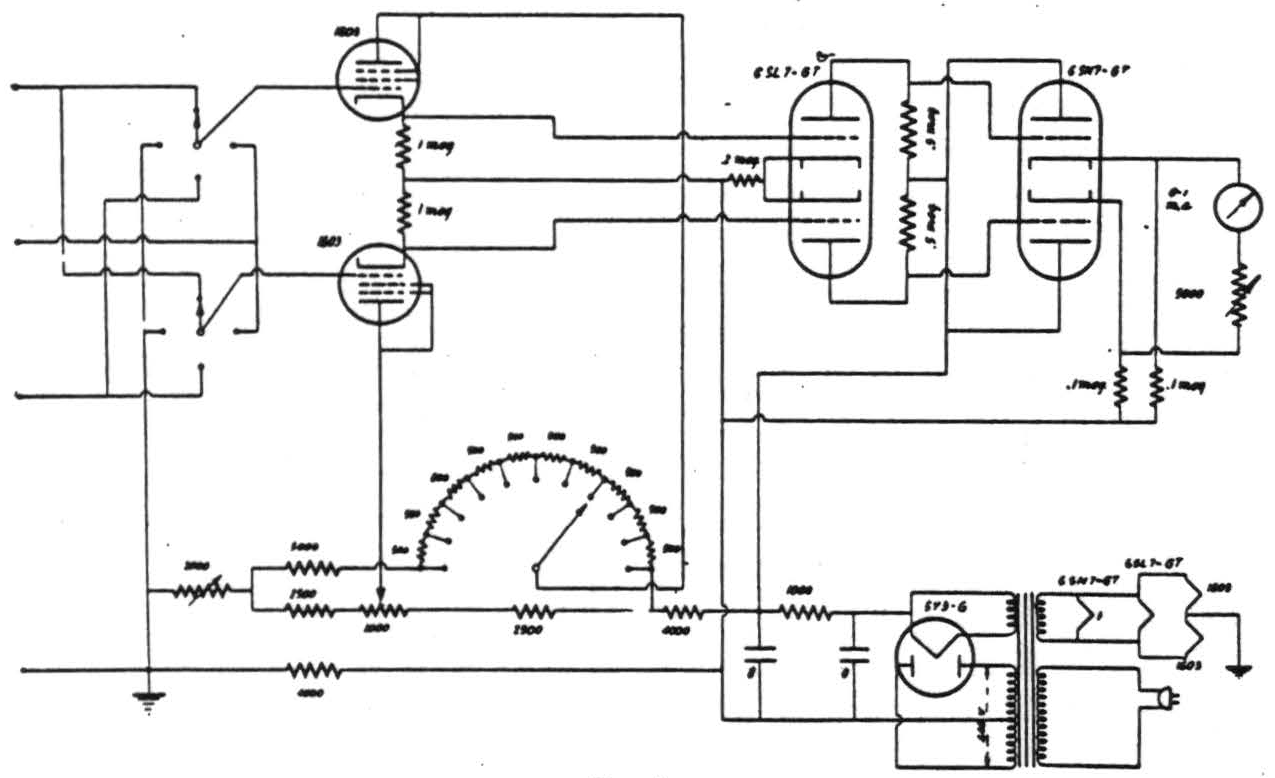
Figure 1. Circuit of the voltmeter, featuring cathode-coupled input which draws less than 10-12 amperes. The gain and the voltage-divider are so adjustable that the full-scale deflection of the meter, and also each step of the decade resistor correspond to 0.1 volt input. A d-c amplifier may be connected to the cathodes of the 1603's or of the 6SN7-GT for the purpose of recording.
Using any noble-metal (usually gold-plated platinum) as an electrode of 1.5 mm2 in conjunction with a vacuum-tube voltmeter (see Fig. 1) drawing less than 10-12 amperes, it is possible to measure redox potentials in vitro. The same procedure has been applied to the cerebral cortex in vivo.
One noble-metal, one glass and 2 Ag-AgCl saline-wick electrodes were placed close together on the cerebral cortex of a cat. The EMF between each pair of electrodes was measured and, on some occasions, recorded continuously, on an Offner crystograph at .5 cm per min. The vacuum-tube voltmeters, each having differential input, were employed synchronously. The EMF of the glass electrode against either wick electrode was correlated with a pH by standardization in buffers of known pH, and the EMF of the noble-metal electrode against either wick electrode was correlated with the redox potential of systems of known composition of oxidant, reductant and pH.
We wish to confine our present note to a few of the results obtained to date.
- When the effect of ether used for operation has passed off in cats curarized with dihydro-β-erythroidinehydrobromide2 by continuous infusion, and under constant artificial
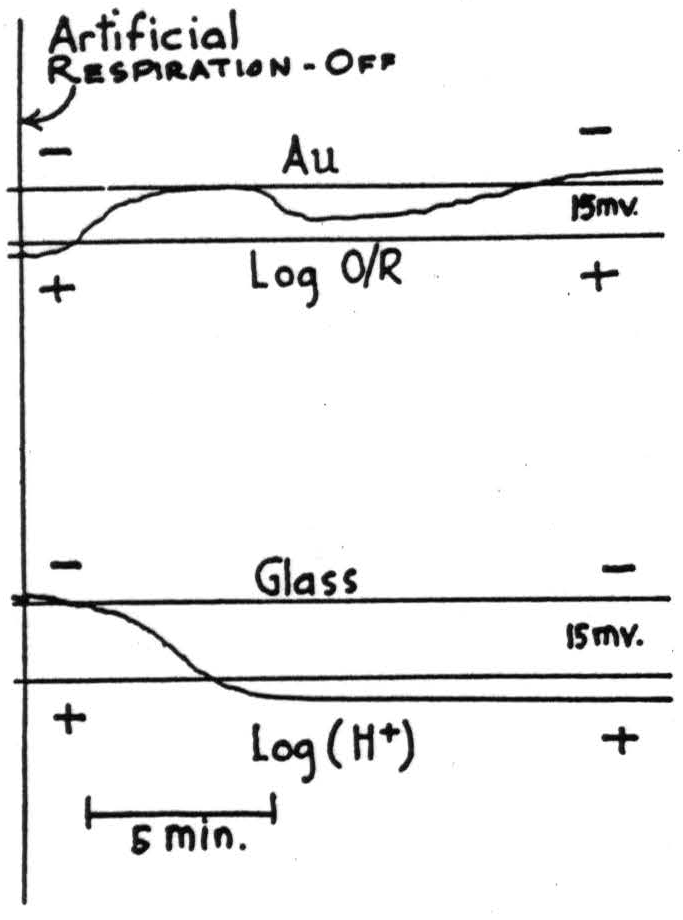
Figure 2. Cortex of cat under dihydro-beta-erythroidine hydrobromide. Cryatographic records of amplified voltages from (top) noble-metal and (bottom) glass electrodes compared with nonpolarizable silver chloride salt bridge electrodes, showing effect of cessation of artificial respiration. Scale approximately linear throughout central 15 millivolts indicated by lines on both records. Note principal deflections are in opposite directions.
respiration, the EMF of glass and noble-metal were practically constant.
- The DC voltages between wick electrodes under the conditions of these experiments were less than one millivolt, which was one-half the least count of the instruments.
- The EMF of noble-metal electrodes, platinum, gold, tantalum or carbon, were not significantly different and were most frequently between .14 and .15 volts positive to the theoretical hydrogen electrode feferred to which the Ag-AgCl wick electrode is .27 volts positive.
- Alteration of composition of respired gas from 100% to 4% O2 in N2, although it alters the O2 tension of cortex by a factor of 4, did not affect the EMF of either glass or noble-metal.
- Intravenous injection of glucose was similarly without effect.
- Injection of epinephrine decreased the EMF of both glass and noble-metal, whereas respiration of 5% CO2 in O2 increased both.
- Cessation of artificial respiration produced an increase in the EMF of the glass accompanied by a decrease in that of the noble-metal. When air was readministered at the end of three minutes, both electrodes over-shot their original EMF's and returned to them slowly. (See Fig. 2.)
The equivalence of noble-metals and that they are noble incline one to believe that in vivo as well as in vitro, the observed EMF measures a redox potential. Its value is in the range of the hemochromogens. Since, in computing redox potentials, the log of the. ratio of oxidant to reductant and the log of the concentration of hydrogen ion entei1 with the same sign, changes of both noble-metal and glass in the same direction may only indicate that the system communicating with noble-metals includes hydrogen ion, and one cannot conclude that the change involves any other significant alteration in the ratio of oxidant to reductant. However, the large changes seen upon cessation of respiration are opposite in sign, implying a great change in this ratio. For this reason, alterations of EMF of noble-metal electrodes may indicate chemical alterations which have escaped other methods of synchronous detection.
Footnotes
For further research:
Wordcloud: Alteration, Amperes, Amplifier, Artificial, Cat, Cathode, Cerebral, Cessation, Changes, Constant, Continuous, Correlated, Cortex, Direction, Draws, Effect, Either, Electrode, Emf, Fig, Glass, Hydrogen, Illinois, Increase, Injection, Input, Known, Measure, Metal, Noble-Metal, Noblemetal, Opposite, Oxidant, Ph, Placed, Positive, Potentials, Ratio, Records, Redox, Reductant, Respiration, Synchronous, System, Vacuum-Tube, Vivo, Voltmeter, Volts, Wick, Wish
Keywords: Voltmeter, Cortex, Noble-Metal, Potentials, Evolution, Systems, Glucose, Civilization, Introduction
Google Books: http://asclinks.live/mmfz
Google Scholar: http://asclinks.live/q08h
Jstor: http://asclinks.live/qscv