CORTICO-CORTICAL CONNECTIONS1[58]
W.S. McCulloch
##
The portion of the cortex to be described is neither an anatomical nor a functional unit. Anatomically, it comprises a large part of the frontal lobe but omits the more anterior portion. Functionally, it includes only about half of that portion of the cortex whose strychninization yields somatic sensation (i.e., the sensory cortex of Dusser de Barenne) and, in addition, cortex anterior, medial, and inferior to it. Yet the selected portion has, as the title implies, one property common to all its diverse constituents: adequate electrical stimulation anywhere within it is followed by an alteration of tension in some muscles; what alteration and which muscles depends upon the site and type of electrical stimulation. As appears in Chapter IX this procedure has been so thoroughly investigated that it now serves to identify each of the principal constituents of this portion of the cortex, and to subdivide several of these in monkey, chimpanzee, and man. Hence it suffices as a criterion for identifying homologous areas. This is of importance to the neurologist or neurosurgeon who wishes to draw inferences concerning man from those experiments on monkey and chimpanzee summarized in this chapter.
When a map of this portion of the cortex is made to show which muscles respond to minimum adequate electrical stimulation at each motor focus, it is at once apparent that all but two of the constituent areas exercise discrete control over specific groups of muscles. Thus there is an easy method of establishing, in this portion of the cortex, that somatotopic subdivision which will be elaborated presently.
If on the map described above are indicated the type of threshold of adequate electrical stimulation and the type and complexity of muscular responses, it can be seen that every constituent of this portion of the cortex has some defining characteristics dependent upon the concentration, caliber, and course of its efferents. The constituents so distinguished correspond to those cytoarchitectonic areas described histologically in Chapter II, except that in monkey and chimpanzee we must distinguish two areas — herein called 4q and 4r — which lack in these animals those cytoarchitectonic differentiations serving to distinguish areas 4y and 4a in man. No two of these areas have the same intra- and inter-areal cortico-cortical connections. Figure 79 shows the entire region under discussion - in monkey (a), chimpanzee (b), and man (c).
Somatotopic Subdivision
The anterior part of the central section is motor to most somatic muscles. It consists in the monkey and chimpanzee of areas 4q, 4r, 4s, 6, and 44. In all of these except 4s and 44 it is possible by appropriate stimulation to distinguish major subdivisions for leg, arm, and face, which are separated by narrow regions for the trunk and neck respectively. By other means these subdivisions can be identified in the postcentral portion of the central sector. As shown in Chapter V, each of these somatotopic subdivisions receives impulses from the lateral thalamic nuclei mediating sensation of the corresponding portion of the soma. Moreover, each of these subdivisions sends impulses back to the corresponding thalamic nucleus or nuclei.
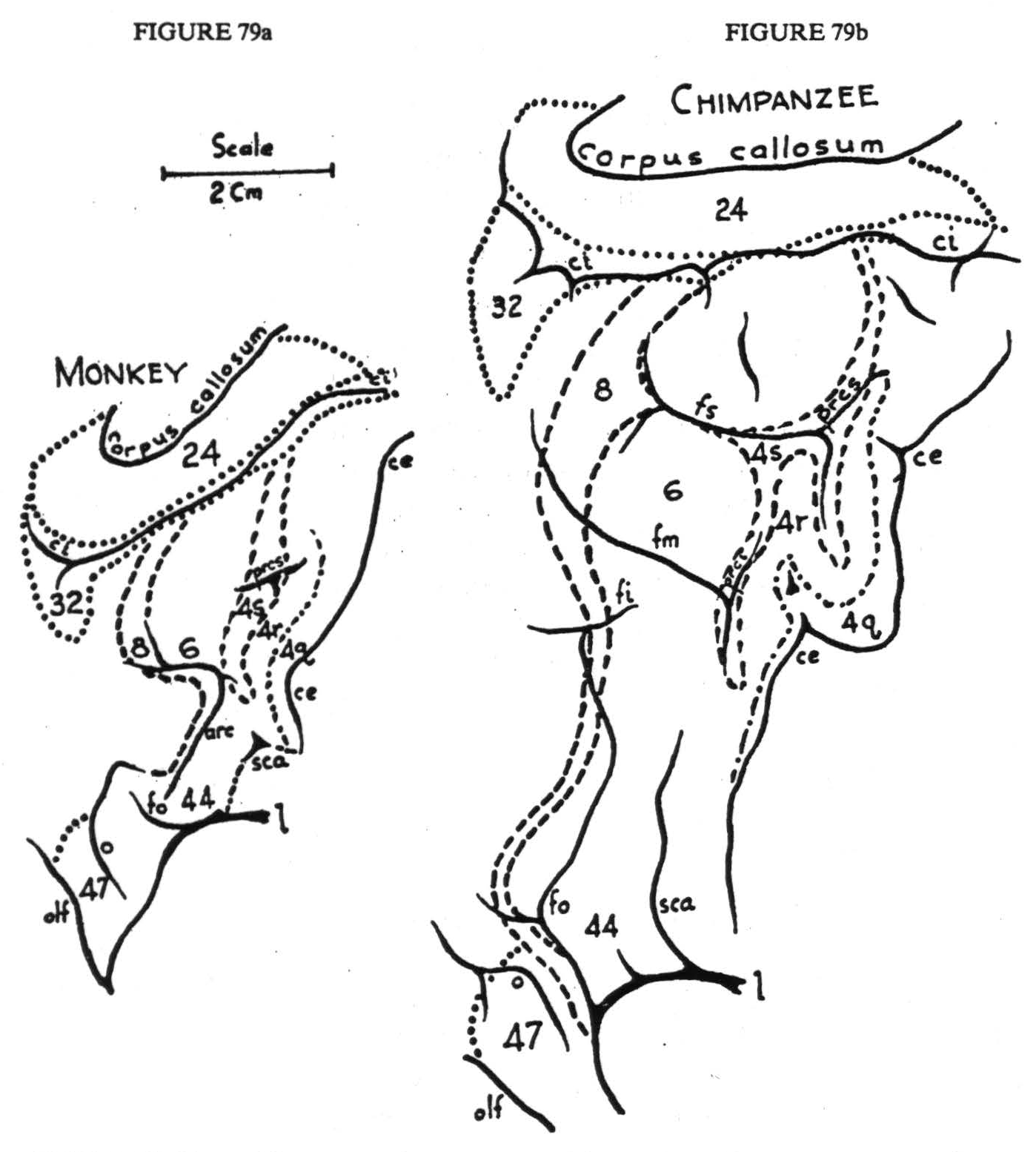
Figure 79. Maps of the precentral motor cortex of the monkey, chimpanzee, and man, drawn to the same scale. To show continuity of the cortex, the area is unrolled: medial aspect appears inverted above; lateral aspect, center, orbital aspect, below. For significance of numbers, see text.
This is schematized in Figure 80. In 1916 and 1923 Dusser de Barenne and Sager showed that in cat and monkey excitation of each of these “sensory” lateral thalamic nuclei, either directly, by local strychninization within it, or indirectly, by local strychninization
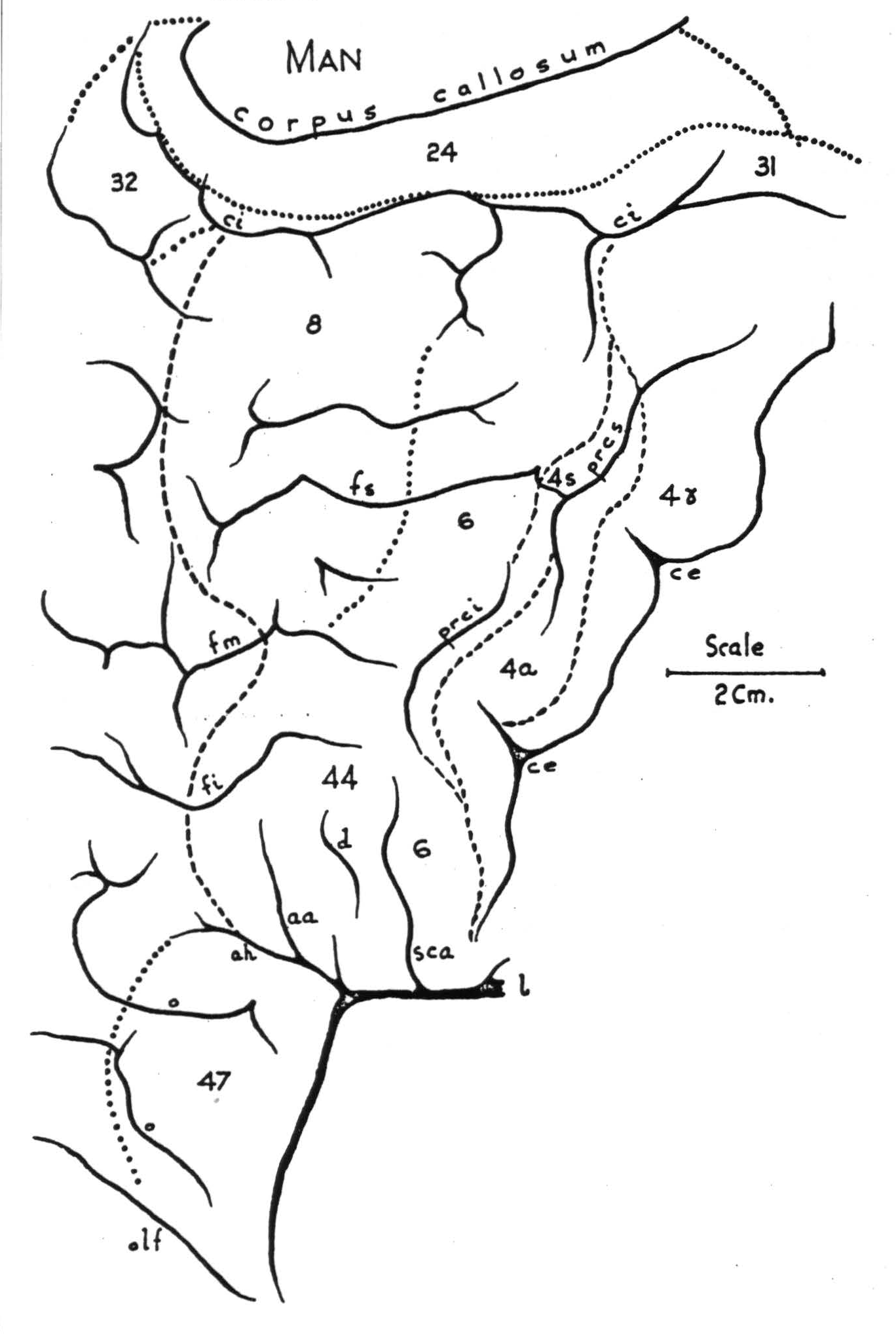
Figure 79c. (For explanation, see preceding page.)
within the corresponding subdivision of the sensory cortex, results in the clinical manifestations of paraesthesia, hyperalgesia, and paralgesia of the corresponding parts of the soma. Although the monkey refers the sensations bilaterally, and more acutely to distal than to proximal portions, no other part of the body is involved unless the strychnine crosses the boundary between somatotopic subdivisions of the sensory cortex or invades lateral thalamic nuclei belonging to more than one subdivision. The importance of this in understanding sensation will appear later. It is described here to indicate that the sensory as well as the motor functions of areas 4q, 4r, and 6 show sharply delineated somatotopic localization.
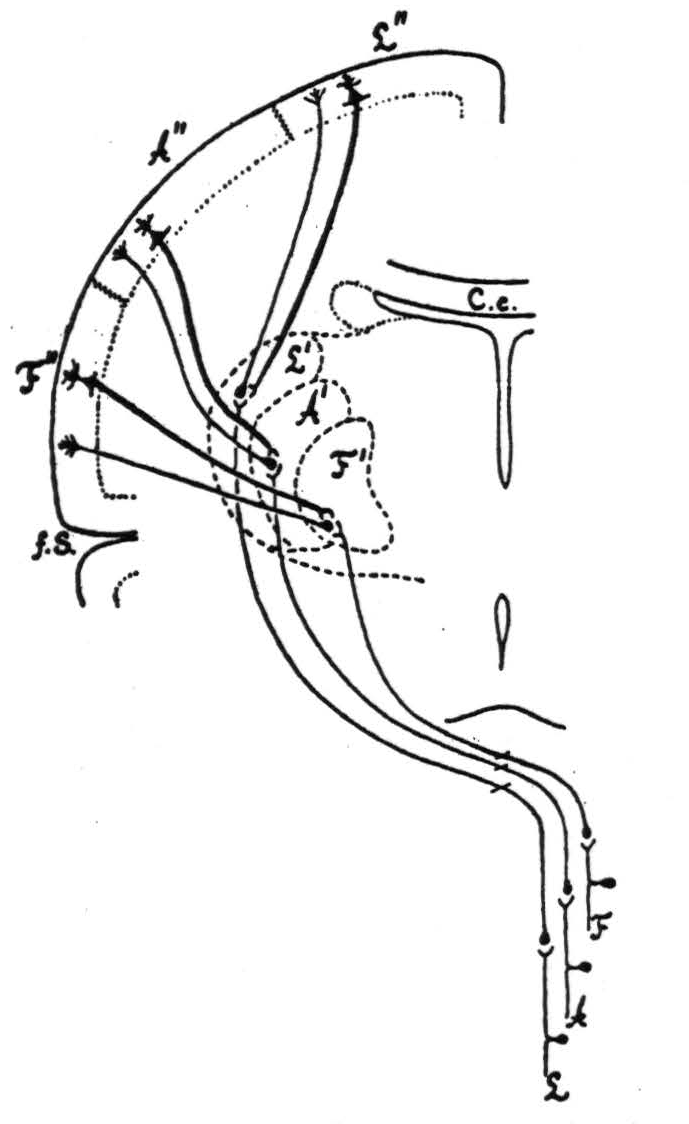
Figure 80. Connections of sensory cortex and lateral sensory thalamic nuclei as revealed by strychnine and electrical record.
While adequate threshold stimulation of any particular "motor" focus elicits a unique muscular response, there is an interplay of excitation between any two motor foci belonging to any one subdivision. Except when the interval between excitations is too long, or the motor responses are antagonistic, this interplay is such as to produce what has been called “secondary facilitation” — a term used to describe either of two distinct phenomena: if two points are selected such that excitation of the first point, a, evokes a motor response, A; and excitation of the second point, b, evokes B; and if a and b belong to the same subdivision and if A and B are not antagonistic, then appropriately timed successive stimulation of a and b will cause either (1) a repetition of A when b is stimulated, or (2) an exaggeration of the response B. The first of these types of “secondary facilitation” (ab-A) can be obtained even when the point subsequently stimulated lies in certain regions of the sensory cortex from which no primary response can be elicited. This (ab-A) has been employed by many observers to obtain motor responses from the post-central sensory cortex. It may involve cortico-cortical connections but depends chiefly upon excitation persisting in the subcortical structures affected by the first stimulation, for b-A can be demonstrated after severance of all cortico-cortical connections between a and b. It is therefore not surprising that ab-A is more easily elicited if both points lie in the same subdivision, for these must project to the same or closely related portions of the spinal cord.
Figure 81 shows the second type of facilitation. This second type of “secondary facilitation” (ab-B) can be elicited even if the point antecedently stimulated yields no motor response. It (ab-B) is invariably associated with an electrical change in the cortex at b (the “facilitated” focus), and ab-B fails when all cortico-cortical connections of a and b are severed. The response ab-B is elicited most regularly at a somewhat longer interval than ab-A, and ab-B can be demonstrated regularly when a exhibits extinction of motor response. Finally, as will appear later, there is reason to believe that the cortico-cortical connections from area 6 to area 4 are large and numerous, while in the reverse direction, from area 4 to area 6, they are wanting; and in this case, if a is in area 4 and b in area 6, only ab-A appears, whereas if a lies in area 6 and b in area 4, only ab-B has ever been described. Thus the occurrence of ab-B can be taken as evidence of cortico-cortical connections. In the monkey's areas 4q and 4r, ab-B can be demonstrated from any point to any other point, provided a and b lie in one and the same subdivision, however remote, but not if a lies in one subdivision and b in another, although a and b be separated by only one or two millimeters to prevent spread of the stimulating current across the boundary. These findings are exemplified in Figure 81.
They indicate a lack of cortico-cortical connections between the portions of areas 4q and 4r belonging to different subdivisions. This is true of the chimpanzee's as well as of the monkey's cortex.
Figure 81. Oct. 27, 1936. Macaca mulatta. Dial narcosis. This figure shows the existence of secondary facilitation at A from a, 8 mm apart, and the absence of facilitation across the functional boundary between the leg and arm subdivisions, although A and L are only 3 mm. apart.
The foregoing findings are all obscured by any procedure which produces the conditions for self-sustained electrical afterdischarge. Thus chloralose anesthesia, stimulation by frequent long electrical pulses, and all cortical insults or other injuries likely to induce convulsions must be avoided. While these self-sustained disturbances of the cortex are propagated over cortico-cortical fiber systems, they are not restricted to these paths but affect contiguous areas of the cortex, eventually inducing the same disturbances in them, even when they are functionally disparate and even after section of the underlying white matter. They may spread with relatively great speed, say 25 cm. per second, involve the entire cortex, become synchronous, and persist for half an hour. It has not been established whether they are transmitted by neuronal paths — such as exist as a feltwork of fibers in certain layers of the cortex — or by chemical changes induced in cells by repeated firing of cells in their neighborhood. Hence, spread of these disturbances through the substance of the cortex cannot be adduced as evidence of cortico-cortical connections of any type. At most, they could only be used to indicate diffuse connections which are already well established by myeloarchitectural and other histological techniques.
To avoid this difficulty, to obtain long and even anesthesia and electrical activity of the cortex resembling normal sleep with relatively little disturbance by afferent impulses, narcosis was obtained by injection of .35 to .45 cc. per kg. body weight of Dial, half the dose given intraperitoneally and half intramuscularly. Except when specifically stated to have been under chloralose, all the following findings were obtained under light or moderate Dial narcosis. The motor responses obtained from the convexity of the cerebral cortex of the chimpanzee are shown in Figure 82.
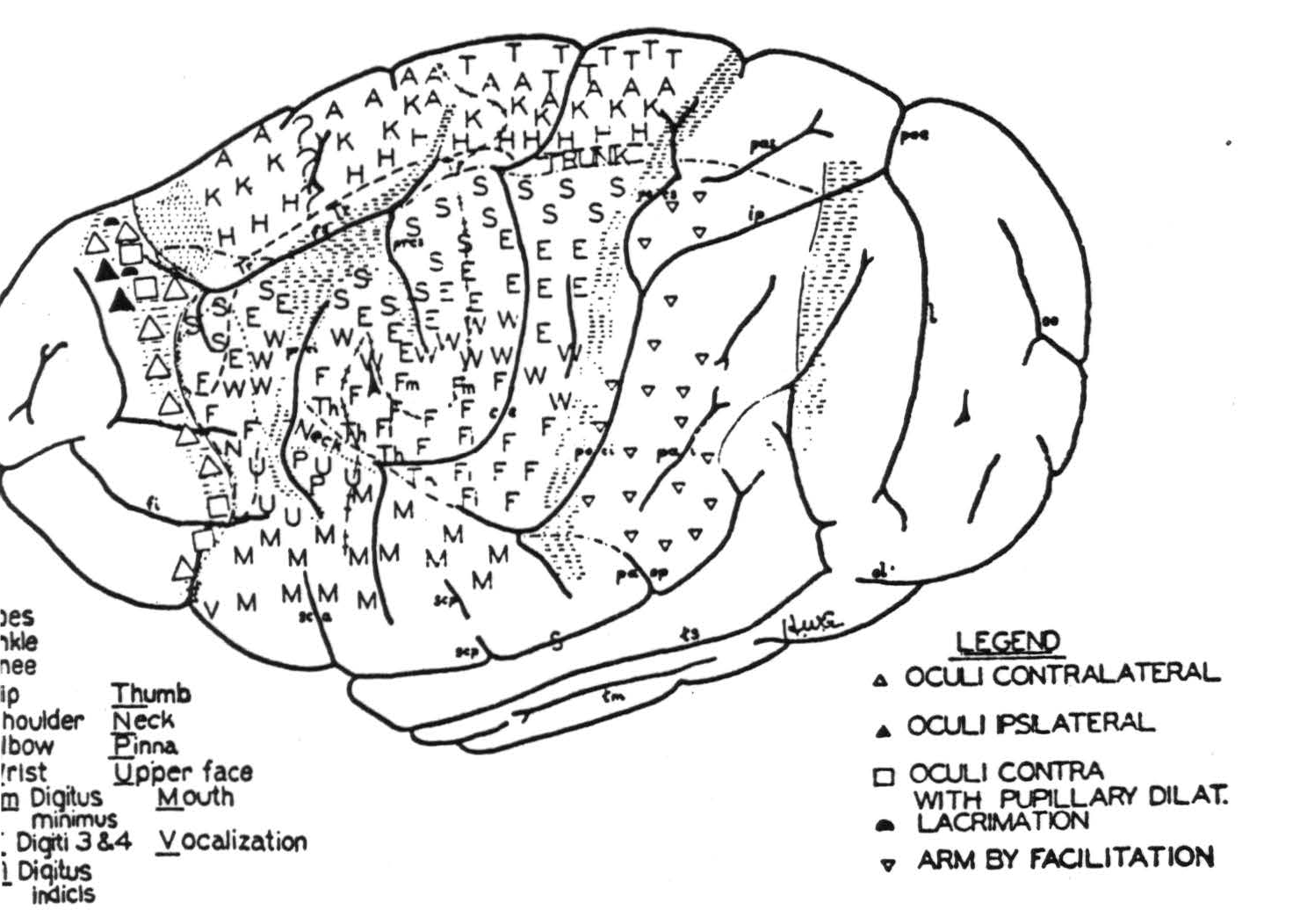
Figure 82.
Under this anesthesia it is possible to map an eye field frontal to area 6 of the sensory cortex. From it, both ipsilateral and contralateral conjugate deviations of the eyes, with and without pupillary changes and lacrimation, have been elicited, as indicated in Figure 82. This is called area 8. In both the monkey and chimpanzee it begins about one millimeter dorsal to the sulcus callosomarginalis as a narrow band running dorsally and slightly frontal at the upper margin of the hemisphere. Thence it descends laterally and widens to the level of the superior frontal sulcus; then narrows and sweeps anteriorly to the inferior margin of the lateral aspect, where it turns medially and occipitally to disappear under the frontal lobe.
On the orbital surface frontal and medial to area 8 lies area 47, known as the area orbitalis agranularis, stimulation of which yields cessation of respiration in inspiration with the vocal cords abducted. Walker, evidently influenced by Brodmann's figure for the lemur or hapale, has called this area 13.
Finally, on the medial surface, the frontal portion of the gyrus cinguli is occupied by area 24 which extends from the corpus callosum to within a few millimeters of the sulcus callosomarginalis. Stimulation of this area induces changes comparable to those elicitable from both areas 8 and 4s which will be considered later.
Area 24 is separated from the somatic motor field and from the eye field by a narrow strip of cortex, area 32, which, like the remaining portions of the frontal lobe, has failed to yield motor responses.
Areal Subdivision
Within this region, the subdivisions represented by the functionally defined areas 8, 47, and 24 correspond to cytoarchitectonic entities; whereas in the central sector proper each somatotopic subdivision includes some portion of each of the principal cytoarchitectonic subdivisions. These latter can be distinguished by stimulation. Area 4q begins in and extends forward from the central sulcus as a band, wide in the leg subdivision and tapering to a point in the face subdivision. It is characterized by the elicitation of motor responses which are highly discrete and occur following relatively weak stimulation. Area 4r, lying immediately adjacent to this throughout its length, requires almost twice the strength of stimulation to produce a motor response, but the response is also discrete. Anterior and adjacent to area 4r lies area 4s. This is a narrow band of cortex, stimulation of which leads to a relaxation of existing muscular tension, interruption of an existing after-discharge produced by stimulation of other cortical foci, and, as shown in Figure 83, a suppression of motor response to stimulation of any focus of area 4q or area 4r — an effect having a latency of about four minutes and usually unrepeatable for three-quarters of an hour. This area was first described in the macacus rhesus monkey by Marion Hines in 1936, who showed that its ablation resulted in the development of spasticity. The details of the microscopic characteristics of these areas are to be found in Chapter II.
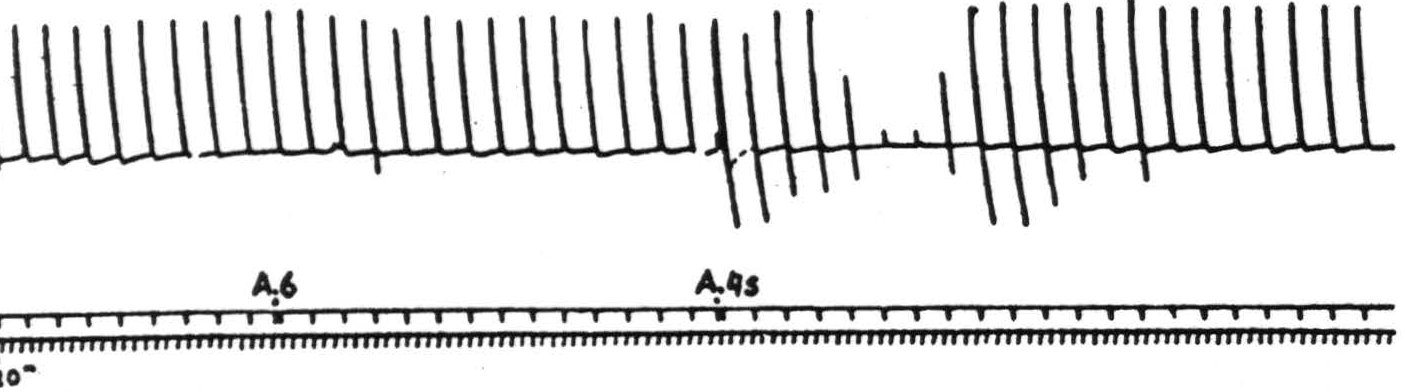
Figure 83. Sept. 27, 1938. Macaca mulatta. Dial. Electrical monopolar stimulation (Thyratron) of A4 focus once every minute (5 sec-0.6 F.-40 per sec.-V.D. 1299) Extension of wrist. Electrical stimulation of A6 focus gives no suppression. Electrical stimulation of A4s focus (6 sec-1 F.-40 per sec-V.D. 7000) gives suppression. Time line = 20 sec.
Anterior to area 4s, and extending below it, lie areas 6 and 44. Excitation of areas 6 and 44 requires slightly stronger stimulation than 4r, or, more specifically, longer electrical pulses, to yield motor responses, and these are apt to involve a larger number of muscles and more proximal groups. Secondary facilitation of the first type (ab-A) occurs readily from any focus of area 4q or 4r to any focus of area 6 of the same subdivision and, by moving the electrode by small steps, can be followed for a considerable distance into area 6 of any adjacent subdivision in the chimpanzee — and even to all parts of area 6 in the monkey. For reasons stated above, this indicates the ramification of fibers descending from area 6. Secondary facilitation of the second type (ab-B) can be demonstrated more readily from area 6 to areas 4q and 4r if the first stimulation occurs in that part of area 6 which yields primary responses belonging to the subdivision containing the focus of area 4q or 4r subsequently stimulated. It can occasionally be demonstrated if the foci lie in different somatotopic subdivisions, but no evidence is available to show that this is mediated by those cortico-cortical fibers whose existence is demonstrable by other means.
To elicit any response from area 8, prolonged stimulation is required, and the motor and glandular responses are slow to appear and slower to disappear. On the other hand, the following three phenomena can be observed: (1) relaxation of existing muscular contractions, (2) holding in abeyance of motor afterdischarge — both (1) and (2) appear and disappear promptly — and (3) suppression of motor response to stimulation of any motor focus of the sensory cortex.
These three phenomena can all be elicited powerfully by stimulation of area 24. Thus there are in the region under discussion three areas, 4s, 8, and 24, which will hereafter be referred to as suppressor areas.
Area 47, lying antero-medial to area 8 on the orbital surface, yields its muscular response to stimuli resembling those required by area 6.
All motor responses depend necessarily on descending systems, which will be found described in other chapters, notably Chapters V and VI. Areas 4q, 4r, 6, and 44 certainly contribute largely to the descending systems of the internal capsule and the pyramids. On the other hand, little, if anything, is known of the motor projection of area 47. Finally, only by local strychninization of the cortex and recording from the corpus striatum have the systems descending to these structures been mapped. Thus, the region under consideration has been shown to project corticotopically; that is, cytoarchitectural areas rather than somatotopic sub-divisions are seen to be represented in the projection. In detail these projections are as follows: areas 4s, 8, and 24 project to the nucleus caudatus (as do areas 2, and probably 19, both of which are also suppressor areas). Area 6 projects to the putamen and to the external segment of the globus pallidus, and areas 4q and 4r to the putamen only. Nothing is known concerning any possible projection from area 44 to the basal ganglia. The obvious scheme seems to be that suppressor areas project to the nucleus caudatus, and motor areas of the sensory cortex project to the putamen and globus pallidus, pars externa.
All these connections are schematized in Figure 84, which indicates approximately the general portion of the nucleus caudatus to which the projection occurs. At this point it must be stated that while all suppressor areas project to the nucleus caudatus, even large lesions of the nucleus caudatus do not prevent suppression of motor response — say from area 4s. This is the more important because suppression of motor response has been shown
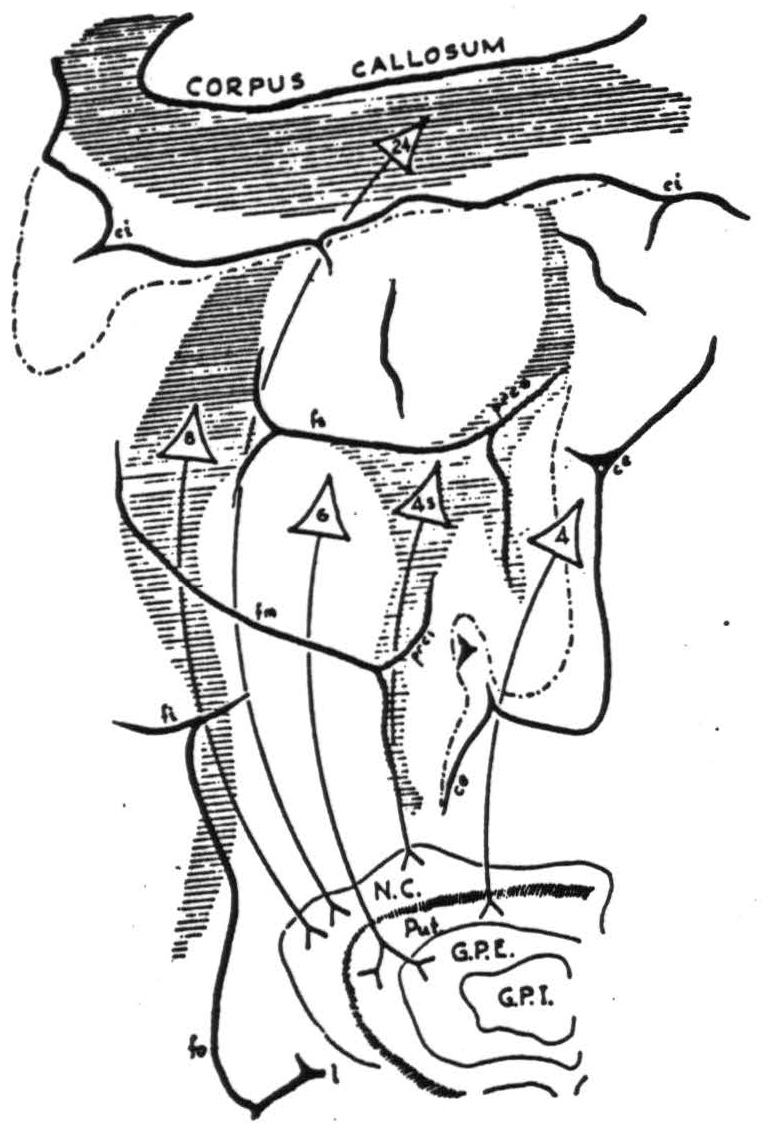
Figure 84. Projections from the precentral motor cortex to the corpus striatum in the chimpanzee as revealed by cortical strychninization and recording of electrical activity in the corpus striatum.
to depend on descending systems, and because another suppression (suppression of electrical activity of the cortex, to be described later) does depend on and is mediated by the nucleus caudatus. At present it is not possible to state what is the descending path mediating the suppression of motor response, although it has been shown that the caput nuclei caudati and the cauda, the putamen, the globus pallidus, the substantia nigra, and the cerebellum are severally not necessary to this suppression. This has been proved only in the monkey. It was accomplished by a series of experiments in which one or another of these structures was destroyed and the suppression of motor response demonstrated within the following eight hours. It can be stated conclusively that the suppression of electrical activity of area 4 by stimulation of area 4s is not necessary to the suppression of motor response to stimulation of area 4 by antecedent stimulation of 4s, because the latter can be demonstrated when the former is prevented by a lesion of the nucleus caudatus.
The suppression of electrical activity of the sensory cortex has been shown to depend on impulses from any of the suppressor areas reaching the nucleus caudatus and thence indirectly interrupting in the ventrolateral nuclei of the thalamus that regular rhythmic oscillation of voltages between sensory cortex and thalamus without which there are no ordinary “spontaneous” electrical waves of the sensory cortex.
Evidence from Physiological Neuronography
The foregoing was intended to familiarize the reader with those criteria by which one can rapidly outline in any one experiment on a living brain those somatotopic subdivisions and cytoarchitectonic areas whose connections form the subject of this chapter. As the author has had no experience with either histological or histopathological techniques he can only refer the reader to Chapters II, IV, V, and VI, which include the principal evidence concerning these connections as revealed anatomically in the dead brain. The evidence on which the present chapter rests was produced by what Dusser de Barenne has christened “Physiological Neuronography.” His life's work has demonstrated that strychnine locally applied acts only where synapses are present on neurons and causes disturbances which are propagated only in the direction of normal conduction — not antidromically. These disturbances appear in records of electrical activity as large, sudden voltages which can be recorded at the site of strychninization and from all regions to which the strychninized neurons send axons or collaterals. At the site of strychninization these sudden transient voltages are many times greater than the ordinary spontaneous activity of the cortex. Cathode ray studies in which the intensity of the spot is made proportional to the first derivative of its displacement, but the intensification is slightly delayed, indicate that these disturbances are composed of almost synchronous discharges of many cells which, together, produce the observed spikes in the record of the voltage. The size of the spike in any other place to which the disturbance is propagated must be determined by the number and synchronicity of the axonal disturbances reaching that place from the cells fired synchronously at the site of strychninization. Under the conditions of the experiments the author has time and again sought for such spikes at regions to reach which the disturbance would have to pass synapses. He has found none. Instead there have appeared only belated low voltage long waves in the record. These are presumably the delayed and temporally dispersed consequences of the pre-synaptic spikes.
Three considerations make it impossible to state exactly the velocity of propagation of the strychnine spikes: first, the length of the axon cannot be readily determined, but it is always safe to assume that it is at least as long as the separation of two cortical “points,” at one of which it arises and at the other of which it terminates; second, these "points" are relatively large, for even with strychninization of only one square millimeter the area of primary spiking is about three millimeters in diameter and the spread of the voltage in the receiving “point” is of at least an equal area; third, and most important, the strychnine spike is not a completely synchronous disturbance but spreads in known ways through the thickness of the cortex. Even so, it is possible to form some estimate of the rate of propagation. Cathode ray determinations indicate a velocity of about 50 meters per second if the initial surface positive wave at the site of strychninization be taken as the time of origin and the beginning of the same wave in the recipient point be taken as the time of termination and the distance is the straight line between the centers of the areas. This figure for the velocity of propagation is probably of the right order of magnitude, and it is probably safe to assume that the maximum velocity is not greater than 100 or less than 10 meters per second. In general, higher velocities were calculated when the points were far apart than when they were near together, but it was not certain whether these differences were due to the difficulty in identifying the corresponding times in the two strychnine spikes or whether they were due to either smaller caliber of shorter axons or the failure to take into account the more circuitous path of short “U” fibers. In any case, the time of transit alone does not preclude the possibility of transsynaptic components. However, there are several other considerations that do so. In the first place, if one undercuts the entire sensory cortex by a lesion immediately external to the corpus striatum the distribution of the distant spikes remains the same. If one then strychninizes the second temporal convolution of one hemisphere he can record the spike from the symmetrical focus, although this produces no spike in any other part of the cortex. The first observation indicates that if relaying occurs it must be in the cortex, and the second observation shows no area of the cortex in which relaying could occur. Thus together they exclude “relaying” of the strychnine spike as a necessary factor in propagation, and so strengthen the negative evidence noted above. Finally, it is possible to locate three widely separated cortical points such that strychninization of A causes a spike at B and strychninization of B causes a spike at C, yet strychninization of A, while it may yield a much belated low wave at C, never results in a spike at C.
For all the foregoing reasons it is clear that strychninization of any cortical area causes a synchronous disturbance of cells in the area strychninized, and that this disturbance can be found in all other cortical areas to which it sends a sufficient number of axons. In this manner local strychninization can be used to map the axonal distribution of cells situated in any area; but it must be remembered that if either the cells or origin for a given axonal distribution are too few or too scattered, or the axonal endings in the field in question are too few or too scattered, the method must fail to disclose them. Hence it should be considered that in the following description positive findings are conclusive but not all-inclusive. They indicate the principal and compact cortico-cortical connections. To make this clearer it is well to contrast one experiment under chloralose with the corresponding experiment under Dial. If one strychninizes one square millimeter of arm area in the monkey under Dial, one obtains strychnine spikes in all parts of arm area 4 and in the post-central arm areas (shown in Figure 85), but not in area 6 or any parts of the leg or face subdivisions. If one now circumthermocoagulates the entire thickness of
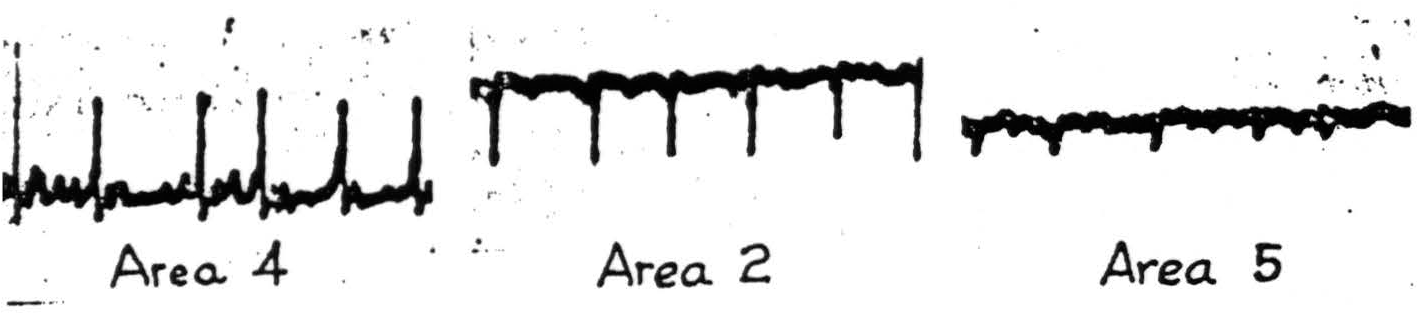
Figure 85. Macaque. Dial anesthesia. Strychninization of area 4. Cathode ray oscillogram 6 minutes later, showing typical strychnine spikes in areas 4 and 2 and small spikes in area 5.
the cortex about the focus strychninized, the distribution of the strychnine spike remains unaltered. It follows from this and the experiment in which the entire cortex was separated from all subcortical structures that the path of these disturbances is downward into the white matter and through it to the post-central areas affected. Rosenblueth and Cannon (1942) showed that under chloralose the strychnine spike described above is complicated by the presence of a much slower disturbance which is also much more slowly propagated, and that this slower disturbance sweeps across all boundaries into area 6, into the adjacent leg and face subdivisions, into all parts of the post-central cortex and even into unrelated regions — its amplitude decreasing as it travels. If now this strychninized focus is circumthermocoagulated, the slow wave disappears, leaving only the strychnine spikes distributed as they are under Dial narcosis. No one doubts the existence of horizontal axons in the substance of the cortex, and it may be through these that the slower disturbance seen under chloralose is propagated. Under Dial — and also without narcosis — the method of Physiological Neuronography fails to indicate their presence. If these provisos are kept in mind no false interpretations are likely to be made of the following findings. It must also be remembered that to procure comparable results the cortex must be exposed with care to insure adequate blood supply and to avoid unnecessary injuries to the pia-arachnoid membrane, and that the surface must be almost dry at the time when and place where a few square millimeters of filter paper moistened with a saturated solution of strychnine sulphate are applied. It needs scarcely be stated that the placing of electrodes and their connection will affect the wave form recorded, and both must therefore always be so arranged as to permit reference of the events recorded to those localities in which they occur. To this end, so-called “triangulation” with several amplifiers having differential input stages is highly desirable.
The results are reported under three general captions: first, the generation and vertical movement of the strychnine spike at its site of origin; second, the distribution of the strychnine spike within the area strychninized; third, the distribution to remote portions of the cortex.
At the Site of Strychninization
When a square millimeter of cortex is strychninized the minute area of surface becomes negative to any remote region, and after less than half a minute there appear on this negativity small negative spikes which steadily increase in amplitude. If at this time an electrode, insulated except at the tip, is plunged into the lower layers of the cortex, these disturbances are recorded as small positive spikes. This is shown in Figure 86. Such wave forms

Figure 86. Monkey (Macaca mulatta). Dial narcosis. Strychnine spikes from postcentral face area: 1, from surface (20″ after strychninization); 2, from depth of 1.35 mm. (1′25″ after strychninization); 3, again from surface (2′10″ after strychninization); 4, again from depth of 1.35 mm. (2′40″ after strychninization).

Figure 87. Macaque. Dial. Local strychninization of an arm 4 focus. Record shows surface potential at the number of seconds after strychninization which are indicated in the diagram.

Figure 88. Section of the motor cortex of area 4, stained by Nissl's method, showing destruction of the layers external to the layer of large and giant pyramidal cells. The animal was killed seven days after local thermocoagulation which was at circa 75 degrees for 3 seconds.
—surface negative, depth positive — are characteristic of disturbances of the superficial layers of the cortex. This is confirmed by Adrian's findings in 1936. They are all that is recorded until the strychnine has had time to reach and excite the deeper layers. When this happens the surface negative wave is preceded by a surface positive wave. Figure 87 shows this development of the strychnine spike. By the method which Dusser de Barenne called “Laminar Thermocoagulation” one can kill the outer layers of the cortex, leaving the deeper layers functional. Figure 88 shows the result of such a laminar thermocoagulation. If this is done when there are only surface negative waves, no spike remains; whereas if it is done when the wave has become biphasic the initial surface positive phase remains but the subsequent surface negative phase is gone. This is shown in Figure 89. If the electrode is now plunged
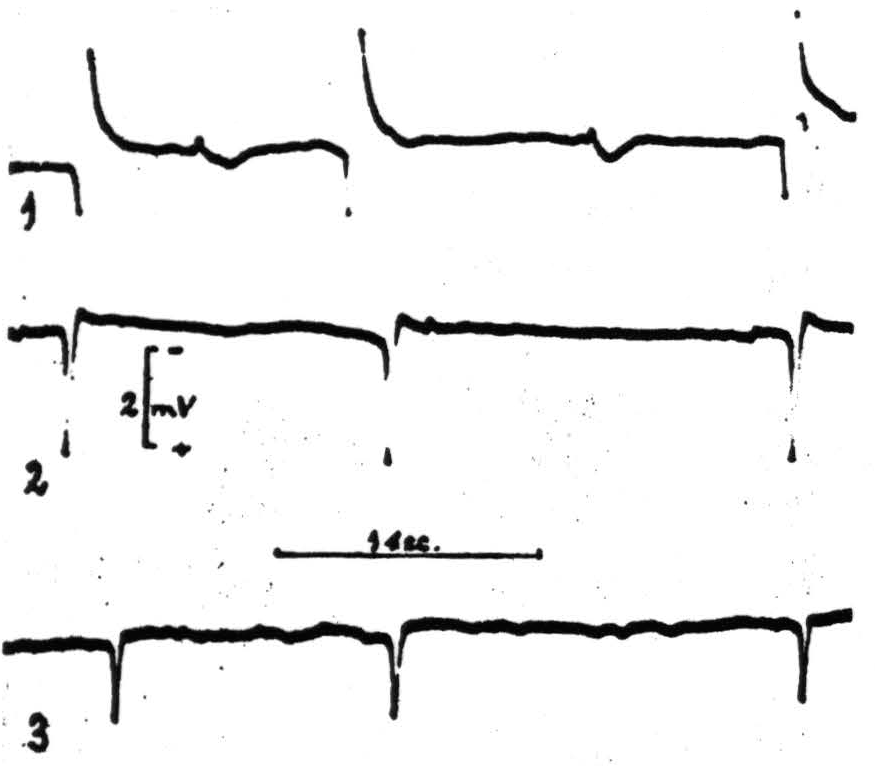
Figure 89. Macaque. Dial. 1, Strychnine-spikes from A6 focus; 2, same, 5 minutes after thermocoagulation of 3 outer layers (TC, 70°C - 3 ½〞); 3, same, 4 minutes after subsequent damage to fourth layer (TC, 70°C - 3 ½〞).
into the deeper layers only the depth negative — i.e., the surface positive wave — is recorded. Therefore this surface positive wave has to be attributed to activity in the depth. If a similar procedure were followed on peripheral nerve, it would be called “rendering a lead monophasic” and one would expect that if the nerve lived long enough the monophasicity of the lead would disappear, and this is what happens in the case of the cortex. Within a matter of hours (five to eight) the diphasicity returns.
There is a second procedure, not hitherto described, invented by Mr. Craig Goodwin of the University of Illinois, and tried out by Dr. Hugh Garol and Mr. John Hamilton for thermocoagulating the deeper layers, leaving the superficial layers of the cortex intact. It depends upon a high frequency current administered to the area through a large chilled electrode. Great difficulty was experienced in obtaining lesions of the desired form and depth, and it may be a long time before the exact conditions for so doing can be prescribed. However, Mr. Goodwin, with Dr. Roseman and Dr. Silveira, has succeeded unexpectedly upon several occasions, and although adequate histological controls are not yet available, their experiments have shown that if the deeper layers are thermocoagulated and any of the superficial layers remain, these layers give only surface negative-depth positive potentials upon local strychninization. Again, the diphasicity returns in some five to eight hours.
Thus these two sets of experiments supplement each other and indicate that, as in peripheral nerve, the disturbance is negative where it occurs and is associated with a positivity at a measured distance of even less than one millimeter.
When the strychnine spike is fully developed in a lightly narcotized cortex the form is triphasic — initial surface positive, subsequent surface negative, and final surface positive, the last being a longer and more widespread disturbance.
Thermocoagulation which abolishes the surface negative phase in no way affects the propagation of the strychnine spike to other cortical points. This is not surprising inasmuch as the early monophasic surface negativity is never associated with propagation. Moreover, when propagation occurs it must, from time studies of the propagation, arise from the initial positive wave under all ordinary circumstances.
At the present time Dr. Silveira is studying (by laminar thermocoagulation, strychninization, and electrical recording) the layers of the cortex giving rise to the cortico-cortical connections. In that undertaking he has already been able to indicate that, at least from certain areas, the efferent impulses continue to go to other cortical areas until the thermocoagulation is sufficiently deep to abolish the surface-positive phase.
Finally, Dr. Silveira has shown that if the most superficial layers of the cortex have been thermocoagulated several days prior to strychninization, the propagation can occur from the second, more widespread, surface-positive phase.
These as yet unpublished studies so complete the picture as to permit the following description of the cortical events under the point of application of the strychnine at the time when the strychnine spike is fully developed. First, there is a discharge of cells in the deeper layers of the cortex initiating both an outgoing impulse to other places and an ascending disturbance. Second, the ascending disturbance involves the superficial structures. Third, the disturbance descends through a greater area of the cortex firing the depths which now can send efferent impulses but cannot reinitiate the ascending disturbance. This is in entire harmony with the picture presented by remote parts of the cortex affected and by axons emerging from the strychninized portion of the cortex. It is moreover, in harmony with all other studies in which cortical potentials have been studied as evoked by impulses afferent to the cortex. Briefly, it is the normal behavior of the cortex — only the stimulation is abnormal — in kind and degree.
Intra-Areal Cortico Cortical Connections
In contrast to the foregoing findings, which are essentially similar in cat, monkey and chimpanzee, the findings hereafter reported show significant differences even between monkey and chimpanzee. As the latter is more comparable to man, it will be described exclusively except to note that in the monkey there is little difference in organization between organization between areas 4q and 4r — and that strychninization of any point of either within any somatotopic subdivision fires both areas throughout
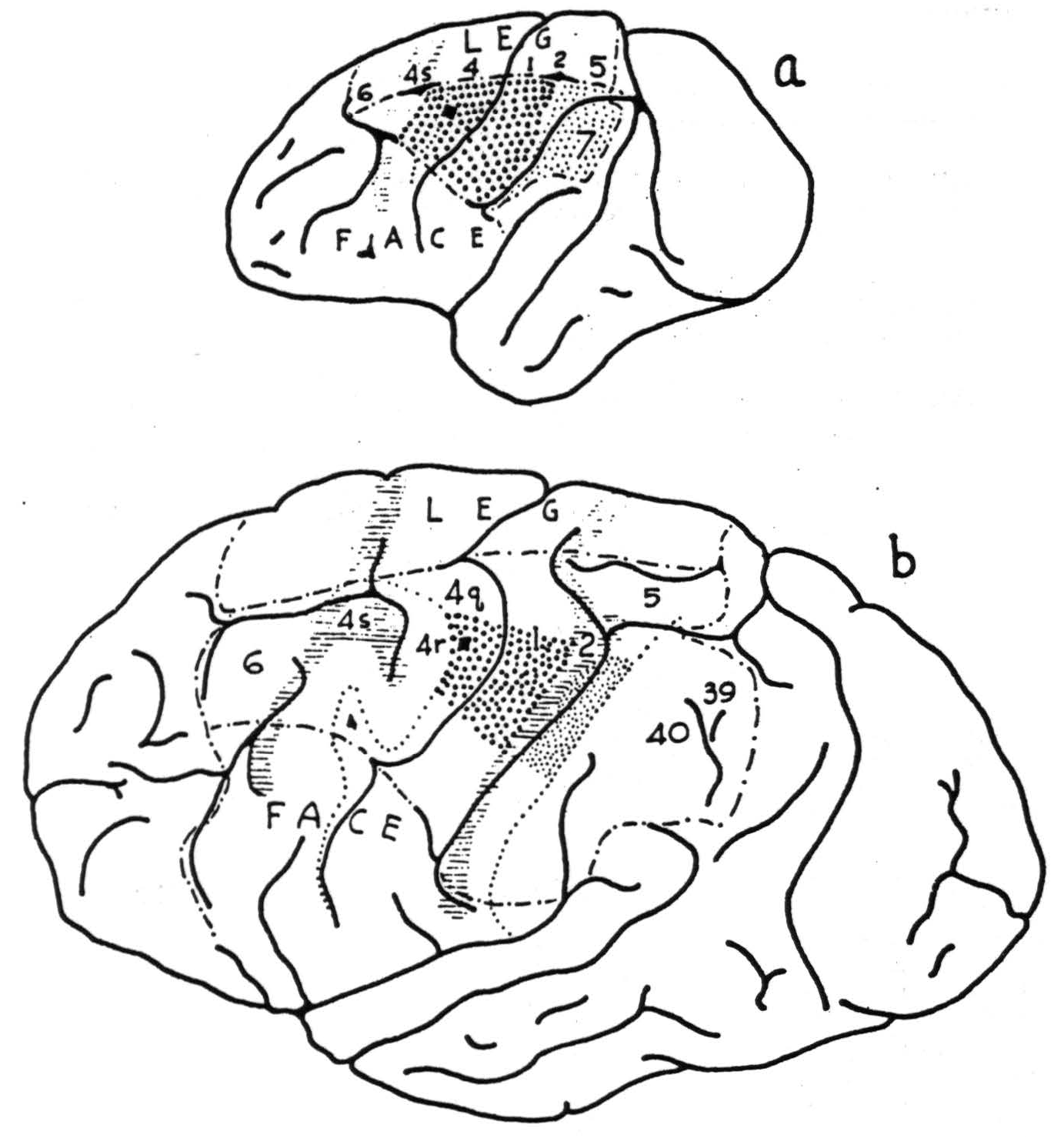
Figure 90. Drawings at the same scale showing comparable local strichninization within area 4 in monkey (Macaca mulatta), above, and chimpanzee (Pan satyrus), below. Note approximate equality of areas fired. Coarse stippling indicates strong firing; fine stippling indicates weak firing.
the subdivision — and that the effects in the post-central sensory cortex of strychninization of 4q and 4r can be differentiated. Figure 90a indicates the effects. Comparison with Figure 90b shows how far differentiation has progressed in the chimpanzee.
Figure 91b locates the areas belonging to the central sector in the chimpanzee.
Area 4q: Strychninization fires area 4q itself only in a small portion of the same subdivision.
Area 4r: Strychninization fires area 4r itself in a slightly larger portion of the same subdivision.
Area 4s: Fires only at focus strychninized, the spread in pickup being no more than can be accounted for by electrical spread of the spike of voltage itself.
Areas 6 and 44: Strychninization in the leg subdivision fires leg 6, trunk 6, and a portion of arm 6. Strychninization in arm 6 fires
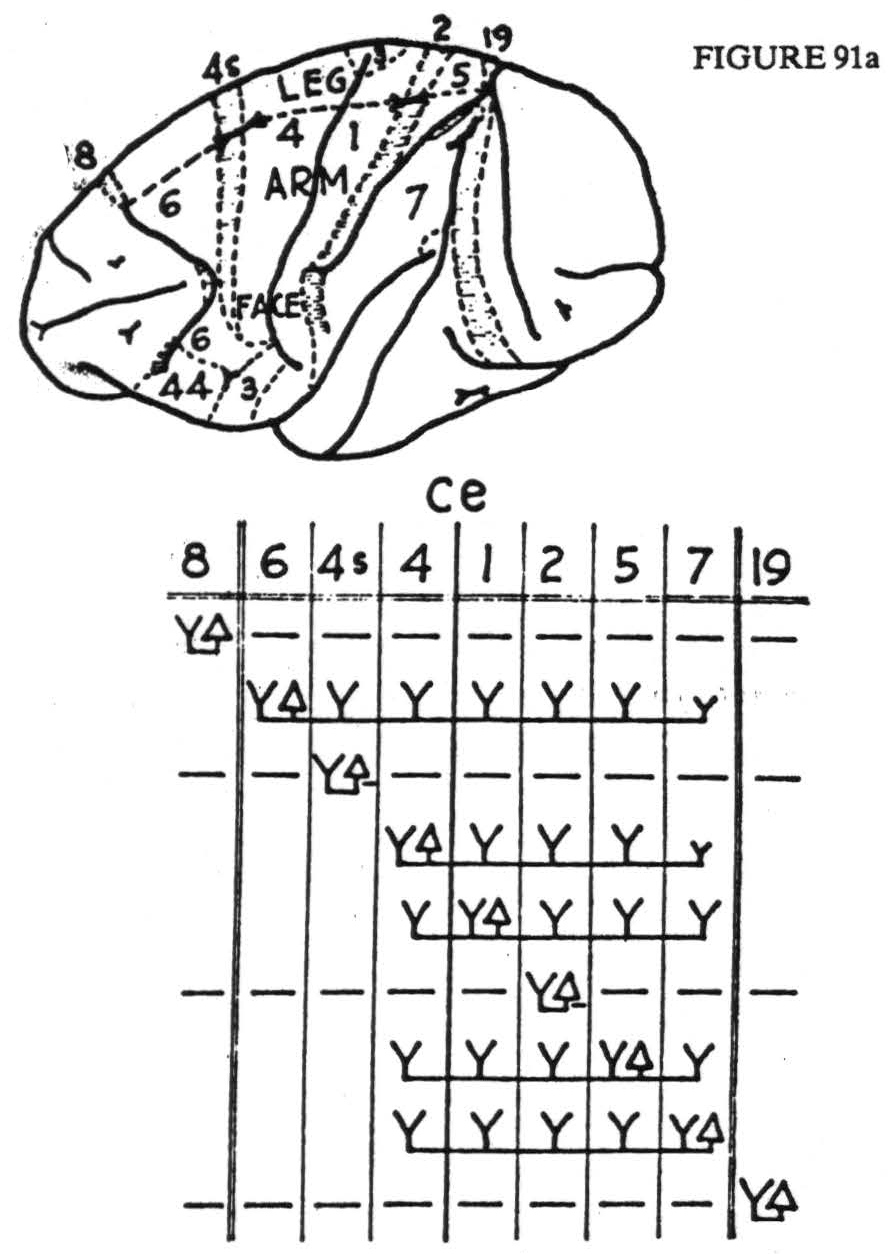
Figure 91. Maps indicate areas of sensory and adjacent cortex, distinguishable by physiological neuronograpny, in monkey (Macaca mulatta), a, and chimpanzee (Pan satyrus), b. Below are diagrams indicating maximal axonal field disclosed by repeated strychninizations in area marked △. Horizontal dashes indicate suppression of electrical activity, Y indicates area fired; ce refers to central sulens; double vertical lines indicate anterior and posterior borders of sensory cortex. For significance of numbers, see text.
trunk 6, arm 6, neck 6, and portions of both leg 6 and face 6. In each case the portions of area 6 fired in subdivisions other than those strychninized seem to depend upon the exact location of the strychnine, but the effects are so interwoven that no comprehensive statement can be made. Face 6 can be divided into two portions, each of which fires itself, but the firing in each case is more restricted than from arm 6 or leg 6. This is all that can be said on this score concerning the chimpanzee — and the reader is referred to Figure 91a for the details of this area in the monkey in which areas 6 and 44 have been distinguished by their efferent cortico-cortical connections. Even so, the cytoarcnitectonic correlation is merely presumptive and no distinction could be made between areas 6bα and 6bβ of the Vogts (1919).
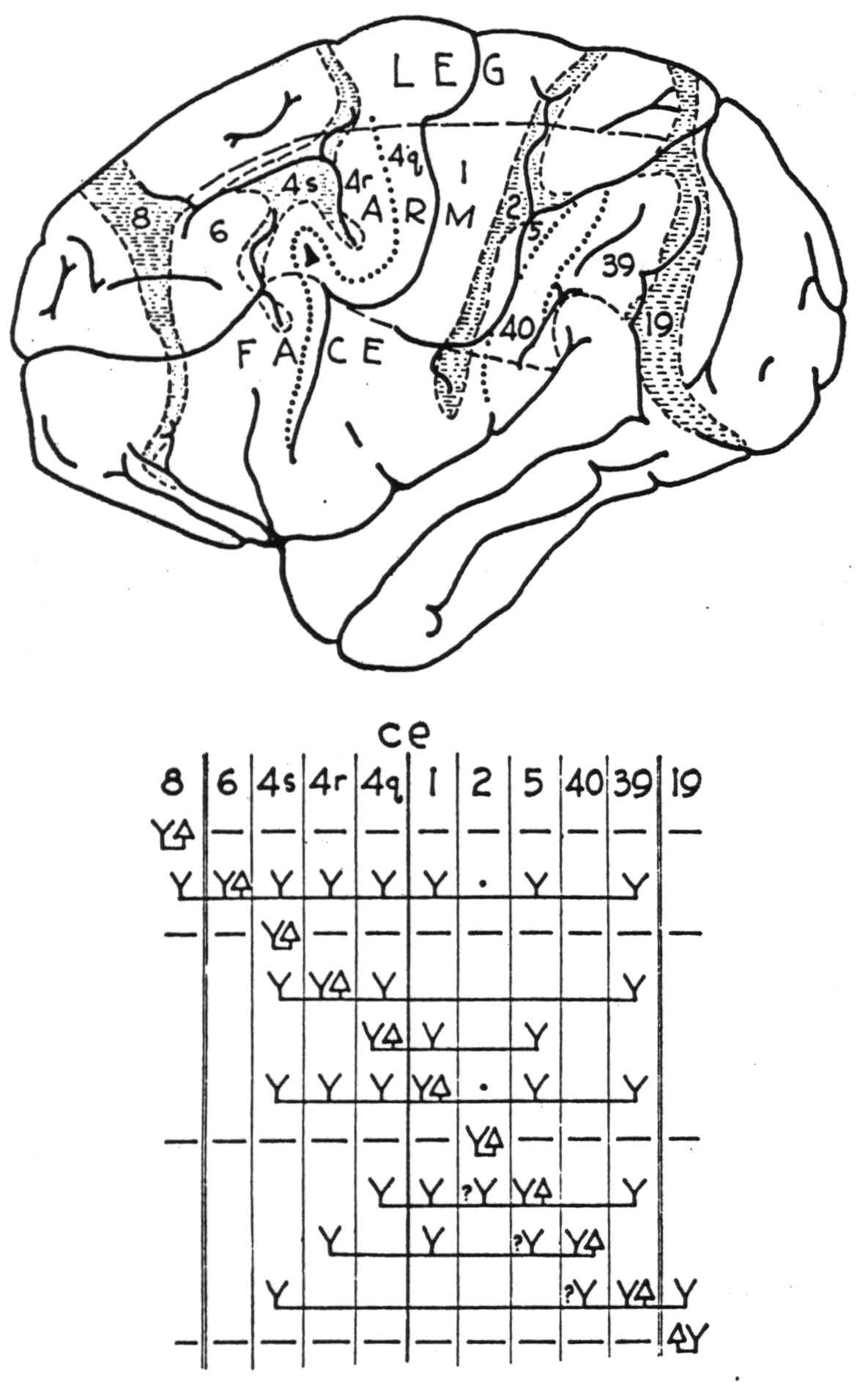
FIGURE 91b. (For explanation, see previous page.)
Area 8: Strychninization of this area, like that in 4s, produces spikes only at the site.
Area 47: Strychninization in this area causes firing within it which is distributed in an antero-posterior band little wider than the strychninization.
Area 24: Strychninization here gives the same extremely local disturbance seen in areas 4s and 8.
Strychninization in the narrow band of cortex separating area 24 from the sensory cortex and from area 8 causes firing throughout that band. Brodmann, in his figure for the monkey, misses all but its anterior end which he there labels area 32, whereas in his figure for man he divides it into two parts: areas 31 and 32. This corresponds most nearly to the areas disclosed in the chimpanzee. Mauss, in his figure of the monkey, calls it area 31 and uses the symbol T. This corresponds to the area as found in the monkey, except for the anterior end which is more nearly like Brodmann's 32 in the monkey. Von Economo and Koskinas so subdivide this area in man (Figure 3b, p.12) that it is impossible to homologize it with the areas disclosed in these studies. Thus Brodmann's figure for man (Figure 2b, p. 11) - areas 31 and 32 — forms the best guide. This area is important not merely because it lies to such an extent within the “motor” area without being “motor,” but because of its afferent cortico-cortical connections which are unique.
Finally, there exists area 3 which, in the face subdivision, becomes precentral. Motor responses have been elicited from this region by most observers. They involve the mouth, tongue, pharynx, and larynx, and can be obtained even after subpial resection of areas 4 and 6. However, this region has been relatively little studied by this method and its boundaries are so ill defined in these experiments that they are scarcely worth reporting. It has, in these studies, always been regarded as belonging to the postcentral region. To work out its detailed organization would probably require an exorbitant number of experiments, for its boundaries lack anatomical landmarks. What little is known of it is indicated in Figure 91a. It certainly fires itself within a somatotopic subdivision, but the attempts to disclose it in the depths of the central sulcus have not yielded sufficiently good preparations to make it certain that firing is restricted to the field, for that could only be established by a large number of negative instances.
Inter-Areal Cortico-Cortical Connections
The inter-areal connections by cortico-cortical axons can best be divided into those which are afferent to and those which are efferent from the area in question. The commissural connections are most commonly symmetrical, or homoiotopic, but the exceptions are important and therefore must also be specified. They will be presented separately, to avoid possible confusion or unnecessary repetition. One other consideration enters here. Since one is forced to assign symbols to areas of the postcentral cortex because these send afferents to the “motor” area, and because these post-central areas lack the criterion of well known motor specificity as a basis of homology, it has seemed best for present purposes to use for them the symbols Brodmann has used for man. Certain of the areas are easily identified, notably 1 and 2, whereas the identification of area 5 is at best tentative, and of area 7, questionable. However, in the monkey the projection of all of these areas to the ventrolateral thalamic nuclei in somatotopic fashion establishes their sensory significance. In the chimpanzee the cytoarchitectonic map is wanting, and in man area 7 fails to appear below the sulcus intraparietalis. In its stead there appear areas 39 and 40 — on the angular and supramarginal gyri, respectively. In the chimpanzee this region projects to the pulvinar instead of to the lateral, sensory, thalamic nuclei. What has become of the large area 7 in the arm and face subdivisions of the sensory cortex of the monkey (see Figure 91a) is unknown. It does seem likely that area 7 crossed the visuosensory band β of Elliot Smith to lie solely in the superior parietal lobule, but the other alternative — namely, that as it developed into areas 39 and 40 it altered its thalamic connections from the lateral nuclei to the pulvinar, seems equally unlikely. However, since the description is to be based on the chimpanzee, one is compelled to regard these areas as 39 and 40 — not as area 7. Figure 91b is best used as a guide to the following statements.
Homolateral Inter-Areal Connections
Area 4q: Receives cortico-cortical impulses from areas 4r, 6, 1, 5, and in the leg subdivision from area 7; sends impulses to areas 1 and 5.
Area 4r: Receives impulses from areas 6 and 1, and in the arm subdivision from what is here called area 40; sends impulses to areas 4s, 4q, and what is here called area 39.
Area 4s: Receives impulses from areas 6, 4r, 1, and 39, but sends impulses only to area 32. The last confirms the anatomical finding of a tract running from 4s into the vicinity of the sulcus calloso- marginalis described in Chapter V.
Area 6: No cortico-cortical afferents have been discovered. In the leg subdivision and in the arm subdivision, area 6 sends impulses to both leg and arm subdivisions of areas 4s, 4r, 4q, 1, and 5, into area 39 from the arm subdivision, and into the posterior part of the superior parietal lobule from the leg subdivision. In the face subdivision area 6 sends impulses to the free subdivision, but the detailed evidence in the chimpanzee is inadequate for a full statement. The reader is referred to Figure 91a for the analysis in the monkey.
Area 44: Nothing definite is known.
Area 8: No cortico-cortical afferents have been found, and no cortical efferents except to area 32 and, from one part, just anterior to arm area 6, to area 18.
Area 47: No cortico-cortical efferents have been discovered. Its cortical efferent systems run, via the fasciculus uncinatus, to area 38, which is the temporo-polar area.
Area 24: No cortico-cortical afferents have appeared, but they have not been sought exhaustively. Its cortico-cortical efferent fibers run into areas 31 and 32.
Areas 31 and 32: These areas receive impulses from areas 19, 2, 4s, 8, and 24 — i.e., from all suppressor areas hitherto found. If areas 31 and 32 be considered two, each is afferent and efferent to the other, but it seems more sensible, on the basis of myeloarchitecture, to consider it as one area, as Mauss did in the monkey.
Commissural Cortico-Cortical Connections
It seems fairly certain that all the interhemispherical cortico-cortical connections of the region of cortex under consideration pass through the corpus callosum, not through the anterior commissure. The only possible exception involves area 47, on whose interhemispherical connections neither the work reported here nor any other known to the author has thrown any light. The convexity of the hemisphere has been thoroughly investigated by three methods; first, by lesions and Marchi stains (Chapters IV and V); second, by electrical stimulation and records of electrical response; third, by strychninization and records of electrical response. The first method has been most extensively used by Mettler during the years 1935 and 1936. It was he who coined the term “homoiotopic” to cover the type of projection which is most commonly found throughout — namely, the projection from a region on one hemisphere to the corresponding region of the opposite hemisphere. The author is indebted to him for the suggestion of how widely the projections might be scattered, for this led to application of many more electrodes than would otherwise have been thought necessary and, so, to the discovery of several of these projections which would otherwise have been missed. The second method, employed by Curtis and Bard in 1939 and 1940, had already disclosed all the interhemispherical connections of the upper portion of the convexity of the hemisphere which the author and his collaborators later confirmed. The differences between electrical stimulation and strychninization are that the former may excite axonal terminations causing antidromic firing, and that it may excite any fibers subjacent to the stimulating electrode. These differences may account for the author's inability to confirm all of the findings obtained by electrical stimulation. On the other hand, this difference may be due to the large number of cells that must be fired synchronously to produce what was regarded as a strychnine spike on the opposite hemisphere. Be that as it may, the findings, aside from extensions to areas not previously investigated, differ from those of Curtis and Bard only privatively, and it is probably safe to regard the connections reported here as representing the most numerous and concentrated projections rather than as an exhaustive array. On this same score, the reader should remember that less than one-third of the cortex appears on the exposed surface, and that the depths of the sulci were neither strychninized nor recorded. Figure 92 shows the origins of these systems from the convexity of the hemisphere in the monkey (Figure 92a) and chimpanzee (Figure 92b).
Area 4q: Shows callosal connections which are only homoiotopic, the connections being extremely well localized to the exactly symmetrical motor focus. Moreover, these connections arise only from the representations of trunk, neck, and lower face — i.e., only from motor foci for parts of the soma used almost exclusively bilaterally, i.e., feet, hands, and upper face.
Area 4: The connection is essentially similar to that from 4q.
Area 4s: Sends no interhemispherical connections.
Area 6: All parts of both send homoiotopic and heterotopic connections to most of the sensory cortex of the same somatotopic subdivisions, and in certain instances to points in other subdivisions. In these must be included areas 39 and 40, which, while they are part of the arm subdivision, have been fired from face 6 and leg
Area 8: Sends callosal connections to the contralateral area 18 but to no other part of the contralateral hemisphere. This tract arises from its posterior margin anterior to arm 6.
Area 47: Not yet investigated.
Area 24: Not yet proved to have any such connections, but they have not been definitely excluded.
Areas 31 and 32: Send homoiotopic connections, but it cannot yet be asserted definitely that no heterotopic connections exist, for the studies do not yet exclude all heterotopic possibilities.
The homoiotopic connections mentioned above necessarily indicate that the areas originating also receive homoiotopic connections, but fail to indicate the reception of heterotopic connections from other areas within and without the area under discussion. Hence the receipt of heterotopic connections are listed below.
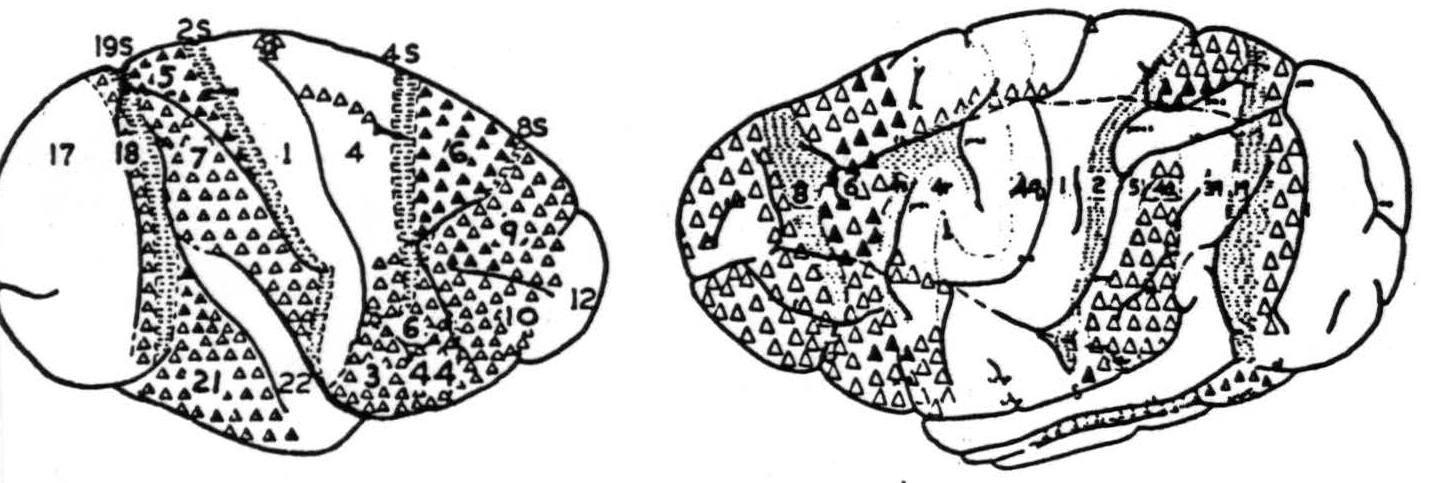
Figure 92. Maps of the convexity of the hemisphere, indicating origins of commissural systems, as revealed by physiological neuronography, in monkey (Macaca mulatta) 92a (left) and chimpanzee (Pan satyrus) 92b (right). For explanation of numbers, see text. △ = Projection to contralateral hemisphere at symmetrical focus only. ▲ = Projection to contralateral hemisphere at symmetrical and other foci. ◬ = Projection to contralaterial hemisphere at symmetrical focus only which remains after section of the corpus callosum
Area 4q: Receives interhemispheric homoiotopic connections from area 4q in the same restricted fashion as that in which it sends them. In addition, many parts, if not all, of area 4 receive heterotopic connections from area 6 and from a small portion of the superior parietal lobule lying inside the sulcus postcentralis superior.
Area 4r: Connections are essentially similar to area 4q.
Areas 4s: Receives no interhemispherical connection, with the possible exception of one from area 6, which has been found only once.
Area 6: Receives only homoiotopic connections.
Area 44: Only insufficient evidence is available.
Area 8: Receives no discoverable callosal connection of any kind.
Area 47: Not yet investigated.
Area 24: Investigated by strychninization of the opposite medial aspect, and no heterotopic firing has been found.
Areas 31 and 32: No heterotopic firing has been found.
This extensive statement of the inter-areal connections is recapitulated in Tables III and IV. In these tables ignorance is
Table III
HOMOLATERAL INTER-AREAL CONNECTIONS
| Area Strychninized | Area Recording | |||||||||||||||
| 31−32 | 24 | 47 | 8 | 0 | 4s | 4r | 4q | 1 | 2 | 5 | 40 | 30 | 19 | 18 | 17 | |
| 31–32 | A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | + | L | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 47 | ? | ? | R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | + | − | − | L | − | − | − | − | − | − | − | − | − | − | − | − |
| 6 | 0 | 0 | ? | 0 | A | + | + | + | + | ? | + | 0 | + | 0 | 0 | 0 |
| 4s | + | − | − | − | − | L | − | − | − | − | − | − | − | − | − | − |
| 4r | 0 | 0 | 0 | 0 | 0 | + | R | + | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 |
| 4q | 0 | 0 | 0 | 0 | 0 | 0 | 0 | R | + | 0 | + | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | + | + | + | F | ? | + | 0 | + | 0 | 0 | 0 |
| 2 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | + | ? | F | + | 0 | 0 | 0 | 0 |
| 40 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | + | 0 | ? | F | 0 | 0 | 0 | 0 |
| 39 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | ? | 0 | + | 0 | 0 |
| 19 | + | − | − | − | − | − | − | − | − | − | − | − | − | R | − | − |
| 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | A | + |
| 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | L |
indicated by ?, definitely established firing by +, and equally well established lack of firing by 0. The reader is again cautioned that the zero does not mean a lack of all connections but merely a lack of sufficient connections to produce an identifiable disturbance. For this reason it summarizes the major inter-areal connections. In those places which represent strychninization within the area recorded, L indicates strictly local firing; R, firing restricted to a somewhat larger portion of the cortex belonging to the same area and same subdivision; A, firing restricted to the area; F, firing of the whole area within the somatotopic subdivision; , the suppression of electrical activity, described below; TNF, trunk, neck, and face only.
There is a second phenomenon which appears at cortical points remote from the site of strychnine when this is in area
Table IV. CONTRALATERAL INTER-AREAL CONNECTIONS
| Area Strychninized | Area Recording | |||||||||||||||
| 31–32 | 24 | 47 | 8 | 6 | 4s | 4r | 4q | 1 | 2 | 5 | 40 | 39 | 19 | 18 | 17 | |
| 31-32 | + | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 47 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| 8 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − |
| 6 | 0 | 0 | ? | 0 | + | ? | + | + | + | 0 | + | 0 | + | 0 | 0 | 0 |
| 4s | − | − | ? | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 4r | 0 | 0 | ? | 0 | 0 | 0 | TNF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4q | 0 | 0 | ? | 0 | 0 | 0 | 0 | TNF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | − | − | ? | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 5 | 0 | 0 | ? | 0 | 0 | 0 | 0 | + | + | 0 | + | 0 | + | 0 | 0 | 0 |
| 40 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 |
| 39 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19 | − | − | ? | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 18 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| 17 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
4s, 8, 2, 24, or 19. It has been called “suppression of electrical activity” (see Figure 93). It begins from 4 to 12 minutes after strychninization and more promptly after mechanical or electrical stimulation of these areas. It consists of a diminution, which may amount to disappearance, of electrical activity of the cortex, first in the vicinity of the area strychninized and then at points more remote, requiring some half an hour to reach the most remote parts of the cortex. By this time the nearer areas have reestablished activity, first as batches of electric waves whose envelope is fusiform, hence called “spindling,” and, later, as normal activity. Figure 93 exemplifies this finding. This suppression is mentioned here to emphasize that, although it is a cortical result of cortical activity, it does not depend upon cortico-cortical connections, for these can be severed without preventing it (see Figure 94). Instead it depends upon cortico-caudatal fibers and is prevented by interrupting them sub-cortically or by pithing the nucleus c audatus. To enhance this dependence of the suppression of electrical activity of the cortex upon descending systems, it is well to note that there is generally a lack of such cortico-cortical connections except to areas 31 and 32, strychninization whereof fails to produce suppression.
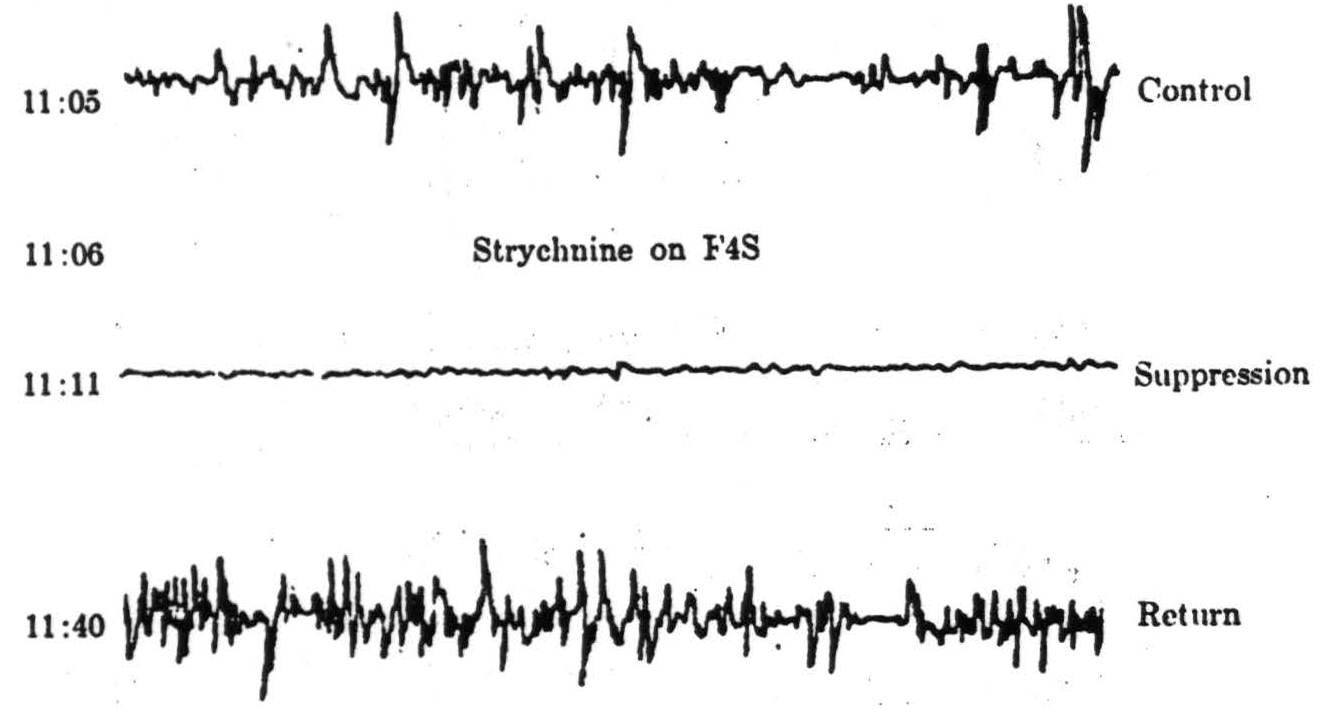
Figure 93. Macaque. Dial. Example of the suppression of electrical activity of area A4 following strychninization of F4s.
One other point must be emphasized to prevent attribution to cortico-cortical connections of phenomena dependent upon descending systems — namely, the appearance of the clinical signs of sensory excitation which follow strychninization of the cortex. The decorticate monkey is too paretic to exhibit any discrete and diagnostic signs, but, if one strychninizes the ventrolateral nuclei of the acutely decorticate cat, the signs of sensory excitation at once appear and are accompanied by “local sign” to such an extent that the unwary examiner is apt to be struck or bitten the instant the hyperesthetic or hyperalegetic portion is touched. Strychninization of the cortex in any sensory area causes firing into the corresponding thalamic nucleus. When, in the monkey, area 6 — be it arm or leg — is strychninized, both arm and leg are fired entire. Hence it is obvious that these clinical manifestations and, therefore, inferentially, the referred sensation, depend solely upon the deeper structures involved and are not even confused by the spread of cortical excitation to other fields. This indicates the necessity of caution in attributing physiological importance to any and all cortico-cortical connections.
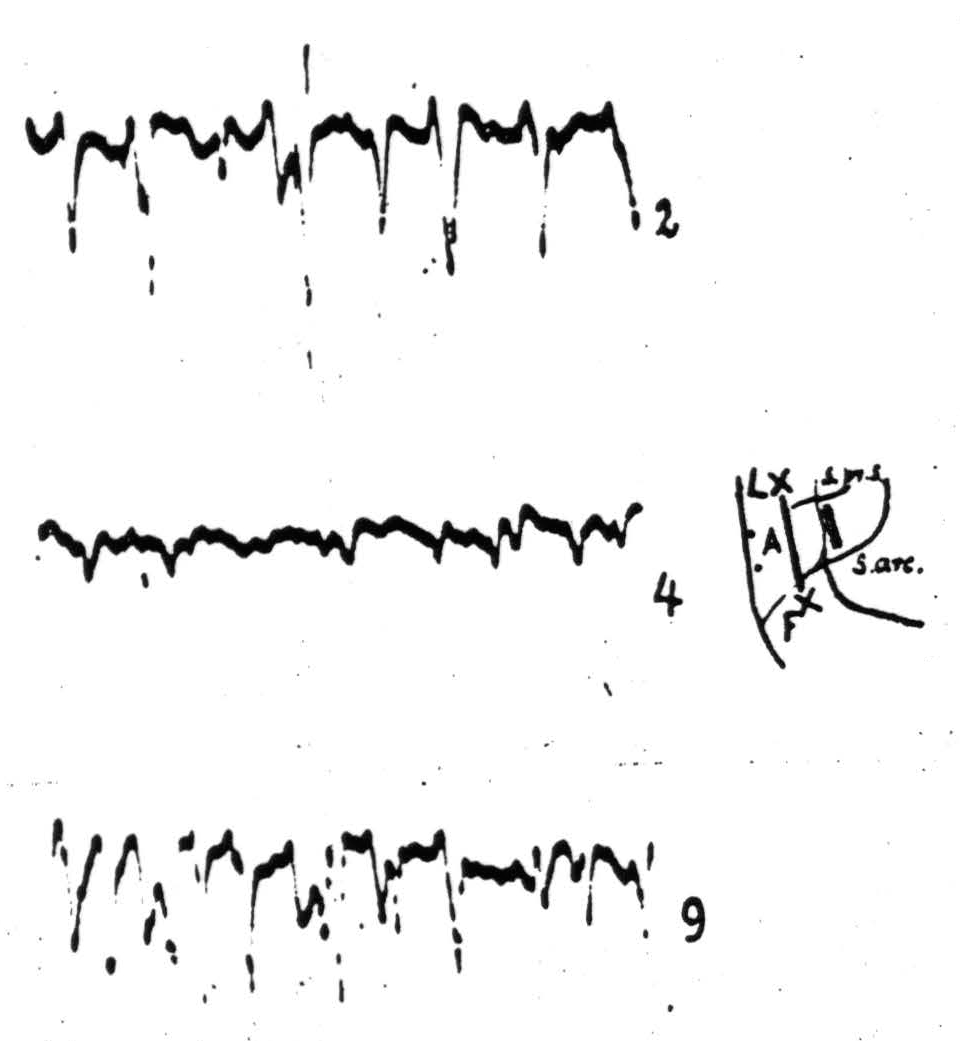
Figure 94. Oct. 18,1937. Macaque. Dial. Electrocorticogram from A4 (:) before (record 2) and after (records 4 and 9) local strycnninization of area 4s (shaded strip). Before the records were taken, a cut (extremities indicated by “x”), 9 1/2 mm. deep, was made between A4 and 4s. Record 4 taken 2 minutes and record 9 taken 12 1/2 minutes after strychnine application to 4s. Note suppression of electrical activity of A4 at height of strychnine action.
To this counsel of caution the corpus callosum is no exception. It has been severed frequently in monkey and in man, but tests of sufficient delicacy or scope to detect any alteration in behavior are still wanting. Severance of the corpus callosum has been shown by Erickson in 1940 to prevent spread of after-discharge from one sensory cortex to the other, and Van Wagenen and Herren reported in 1940 that it had, in certain instances, prevented epileptic seizures of focal origin from becoming generalized. Bishop, in 1937, attributed to the corpus callosum that synchronizing of the electrical waves of the occipital lobes which occurs when these are released from incoming impulses by lesions between the cortex and the lateral geniculate body. Thus this multitude of cortico-cortical connections obviously is capable of determining phenomena in abnormal situations, but the part it plays in ordinary activity and in normal behavior remains a mystery.
There is still less evidence of the role of any other cortico-cortical connections. In a sense this is disappointing for those who wish to think in terms of localized functions where "functions" imply aspects of overt behavior. On the other hand, it is a truism that the significant function of any nerve cell is to be fired or inhibited by those cells which make synaptic contact with it and to fire or inhibit those cells with which it makes synaptic contact. To trace these connections is therefore prerequisite to understanding any function of the brain — for these determine the only functions that can, strictly speaking, ever be localized.
Summary
There are present everywhere in the cortex some cortico-cortical fibers in the gray matter. These serve to propagate incoming disturbances upward through the gray matter and thence downward through a larger area to deeper layers, whence they are transmitted to other parts of the cortex and to deeper structures — to what other parts of the cortex and to what deeper structures depends upon the part of the cortex involved. Local strychninization of the cortex can be relied upon to produce a sufficiently synchronous firing of cortical cells to disclose any of these systems of efferents, provided there is sufficient concentration of them at the site recorded. By this means it is possible to subdivide the precentral motor cortex into five areas (4q, 4r, 4s, 6 and 44) belonging to and intimately connected with other portions of the sensory cortex. Immediately anterior to it lies area 8 which sends impulses back to area 18 — a field which is also "motor" to the eyes. Anterior to this area 8 on the orbital surface this method has revealed area 47 which sends impulses via the fasciculus uncinatus to area 38, capping the temporal pole. Situated within the anterior half of the gyrus cinguli it has disclosed another area which, like areas 4s ad 8, sends impulses to the narrow strip of cortex called areas 31 and 32 and has thus established a pathway to the frontal pole — from these and from all other "suppressor" areas. It has, moreover, disclosed localized homoiotopic callosal connections arising and terminating in areas 4q and 4r, but only from and to those portions of these areas which are concerned bilaterally in ordinary movements. From area 6 which, in a sense, is a motor associational area, it has disclosed the widest distribution of callosal connections, homoiotopic and heterotopic. And, finally, it has failed to disclose any such connections from any of the "suppressor" areas except from area 8 to area 18. These are to be regarded as the chief, but not necessarily the only, cortico-cortical connections arising from each of the above areas. Evidence has been adduced to indicate that the interpretation of the normal function of these connections — other than that of interrelating the activity of the areas in question — is still to be determined. Of all the functions normally demonstrable by cortical stimulation, only one type of secondary facilitation and the spread of cortical after-discharge can definitely be referred to these cortico-cortical connections. The rest, facilitation, extinction, and suppression of electrical activity or of motor response to electrical stimulation of the cortex — even the reference of sensation, like the elicitation of motor response — depend on descending systems.
One relation has probably been understated or obscured by details. This is important when one tries to extrapolate from monkey through chimpanzee to man. So long as the surface area strychninized is held constant and homologous cortical areas are strychninized, the total surface areas of response in the monkey and in chimpanzee are approximately equal (Figure 90). It is as if the concentration of the cells of origin of these systems remained constant for any given region. This means that with expansion of the cortex one would have to expect just such differences as exist between monkey and chimpanzee — namely, that with increase of surface area there appears a greater differentiation in the sense of a greater specificity of distribution. This, in turn, would lead one to expect still greater specificity in man.
Footnote
Acknowledgements
It is only fitting to note here that the author of this chapter is indebted first to J.G. Dusser de Barenne and his collaborators, O. Sager and H.M. Zimmerman; more recently, to H.W. Garol, P. Bailey, and G. von Bonin; and finally to C. Goodwin, J.M. Hamilton, E. Roseman, E.W. Davis, and A. Silveria, who have permitted inclusion of hitherto unpublished observations.
As it has been the author's privilege to observe, repeat, or perform the experiments by which the data in this chapter were obtained, it seemed best to omit references to publications, and merely to include them in the bibliography.
For further research:
Wordcloud: Activity, Anterior, Appear, Area, Arm, Cells, Chimpanzee, Connections, Cortex, Cortical, Cortico-Cortical, Depend, Described, Dial, Disturbance, Electrical, Except, Face, Figure, Firing, Focus, Following, Functions, Impulses, Indicate, Lateral, Layers, Leg, Local, Man, Monkey, Motor, Negative, Point, Portion, Projection, Propagation, Recorded, Region, Response, Sends, Sensory, Shows, Spike, Stimulation, Strychninization, Subdivision, Suppression, Surface, Wave
Keywords: Cortex, Connections, Portion, Areas, Stimulation, Yields, Area, Constituents, Lobe
Google Books: http://asclinks.live/77pf
Google Scholar: http://asclinks.live/96qh
Jstor: http://asclinks.live/rydn