THE FUNCTIONAL ORGANIZATION OF THE OCCIPITAL LOBE12 [49]
G. von Bonin, H.W. Garol and W.S. McCulloch
Introduction
The current views on the organization of the primate occipital lobe are largely based on anatomical evidence, supplemented by experimental and clinical observations.
It was, in point of fact, a physiologist, Munk,(1) who first created the concepts of a “visuosensory” and a “visuopsychic” center. Campbell,(2) in his fundamental cytoarchitectural studies, joined hands with those “who hold the belief that there exist two distinct cortical centers” in the visual sphere, and differentiated in the occipital lobe between a visuosensory and a visuopsychic area, obviously representing the two centers postulated by physiology. The visuosensory area is now generally known as the striate area. Its characteristic structure which gave it its name was dimly recognized more than a century ago by Francesco Gennari(3) as well as by Vicq d'Azyr, and had been described by Meynert,(4) Bolton(5) and others many years before Campbell published his systematic survey of the whole cortex. It has never since been doubted that it forms an anatomical and functional entity. Campbell's visuopsychic area was further subdivided by Brodmann (6) as well as by Elliot Smith (7). Both recognized two areas, concentrically arranged around the striate area (17 of Brodmann) which Brodmann called “occipital” or 18, and “preoccipital” or 19, and Elliot Smith “para” - and “peristriate” area. Brodmann's extensive observations showed that his areas 17, 18 and 19 were present in all grades of primates. Mauss (8, 9) studied the myeloarchitecture of the “cercopithecus,” gibbon and orang, and again recognized these three occipital areas. His boundaries, however, differed from those which Brodmann had indicated. C. and O. Vogt (10) subdivided area 19 of the monkey into a dorsal, 19a, and a ventral, 19b. v. Economo and Koskinas (11) studying the human cortex drew some further distinctions. Within the parastriate area (OBϒ) they recognized close to the striate area a “margo magno-cellularis” OBγ, and they described several modifications of the peristriate area, OA.
The pattern of three occipital areas, with or without further subdivisions, has been widely accepted for all primates. The scheme of Brodmann and Elliot Smith was used for clinical considerations by Kleist(12) (see esp. fig. 225) who considered 18 as the “visuomotor” field and 19 as the “visuopsychic” field. Kleist went even further and localized color recognition in the ventral, reading of words in the middle, and memory for places in the dorsal part of area 19. These attempts at localization have struck many authors as rather extreme(13).
The surfaces of the occipital areas were measured quantitatively by Filimonoff(14, 15) in monkeys, orang and man. His data in mm.2, with some statistical constants computed from his original measurements, are as follows:
| Area | ||||
|---|---|---|---|---|
| 17 | 18 | 19 | ||
| Man | Mean | 2113 ± 30 | 3948 ± 72 | 3890 ± 146 |
| S. D. | 143 | 340 | 687 | |
| Orang | Mean | 1876 ± 39 | 1447 ± 54 | 1417 ± 129 |
| S.D. | 96 | 132 | 315 | |
| Monkey | Mean | 933 | 725 | 609 |
In man 18 + 19 (7838 mm.2) is almost exactly three times as large as 17, in the orang 18 + 19 (2864 mm.2) is not even twice the size of 17, while in the monkey 18 + 19 (1334 mm.2) is still somewhat smaller in relation to 17.
An investigation of the brain of the macaque and of man led Beck (16) to an entirely different pattern. He described not only nine modifications within the striate area of the macaque, but divided the rest of its occipital cortex into 13 subregions of which it is sufficient to say that they were such and so disposed as to cause Beck to deny that the para- and peristriate areas surround the striate area as two rings. Ngowyang (17) confirmed Beck's work on the striate area by pointing out that it was possible to recognize within the human striate area numerous subdivisions. On the basis of but one hemisphere he described 16 “subfields.”
Thus the classical pattern described by the pioneers in cortical architectonics has not remained unchallenged. Even those who accept it in its simple form appear to be unable to give precise and easily recognizable criteria for the para- and peristriate areas. The boundaries are given differently in various maps, and the published pictures are far from always being definite. These discrepancies of definition and the obscurities surrounding the distinction between areas 18 and 19 were so great that the present investigation was begun in hopes of finding physiological criteria in the functional organization of the brain. For only physiological neuronography seemed to promise a solution of these difficulties as well as a meaningful scheme of the occipital lobe.
Experiments.
The experiments were performed upon 12 macaques and 4 chimpanzees. As both primates were similarly investigated and showed the same functional organization, it is superfluous to give detailed accounts separately.
Method.
The experimental procedure is essentially as follows. The animal is anaesthetized with Dial Ciba3 and its head clamped to the table. The skull is then opened, the dura reflected and the exposed brain photographed. An enlarged print for plotting positions is available by the time the electrodes are placed on one hemisphere and are connected 6 at one time to 5 channels of a 6 channel Grass inkwriting oscillograph. A special switch allows any one group of six electrodes to be connected with the oscillograph. The arrangement within a group is the so-called linear one in which successive channels have one electrode in common. The remaining channel is used for a pair of roving electrodes which can be placed each time at the site of strychninization to determine the instants at which disturbances are initiated there and so to facilitate the reading of records. A few square millimeters of filter paper moistened in a saturated strychnine sulphate solution colored with toluidine blue is then applied to the cortex and removed after the “strychnine spikes” have appeared. This usually requires several minutes and the “strychnine spikes” persist for about ¾ of an hour. The next strychninization can then be performed. By keeping the animal's head low to insure circulation and the room warm and wet to delay inspissation of the cortex, an animal can be used for several days and requires only an occasional dose of 0.2 cc. of Dial to maintain narcosis. In this way as many as 50 strychninizations may be performed on one specimen. The locus of each strychninization is recorded on the photographs of the exposed brain along with the site of the electrodes.
Results.
It was indeed possible to define by that method three different areas in the occipital lobe. Following Brodmann's scheme we shall refer to them provisionally in order to shorten the descriptions as areas 17, 18 and 19, although the boundary between 18 and 19 does not lie exactly where Brodmann drew it.
The work on the occipital lobe was facilitated by an initial mapping of the area wherein large, prompt electrical “on” and “off” effects occur in response to light flashed in the eyes4 (Fig. 1). In the macaque this area covers the greatest part of the lateral side of the occipital lobe. Its anterior boundary cuts the superior margin of
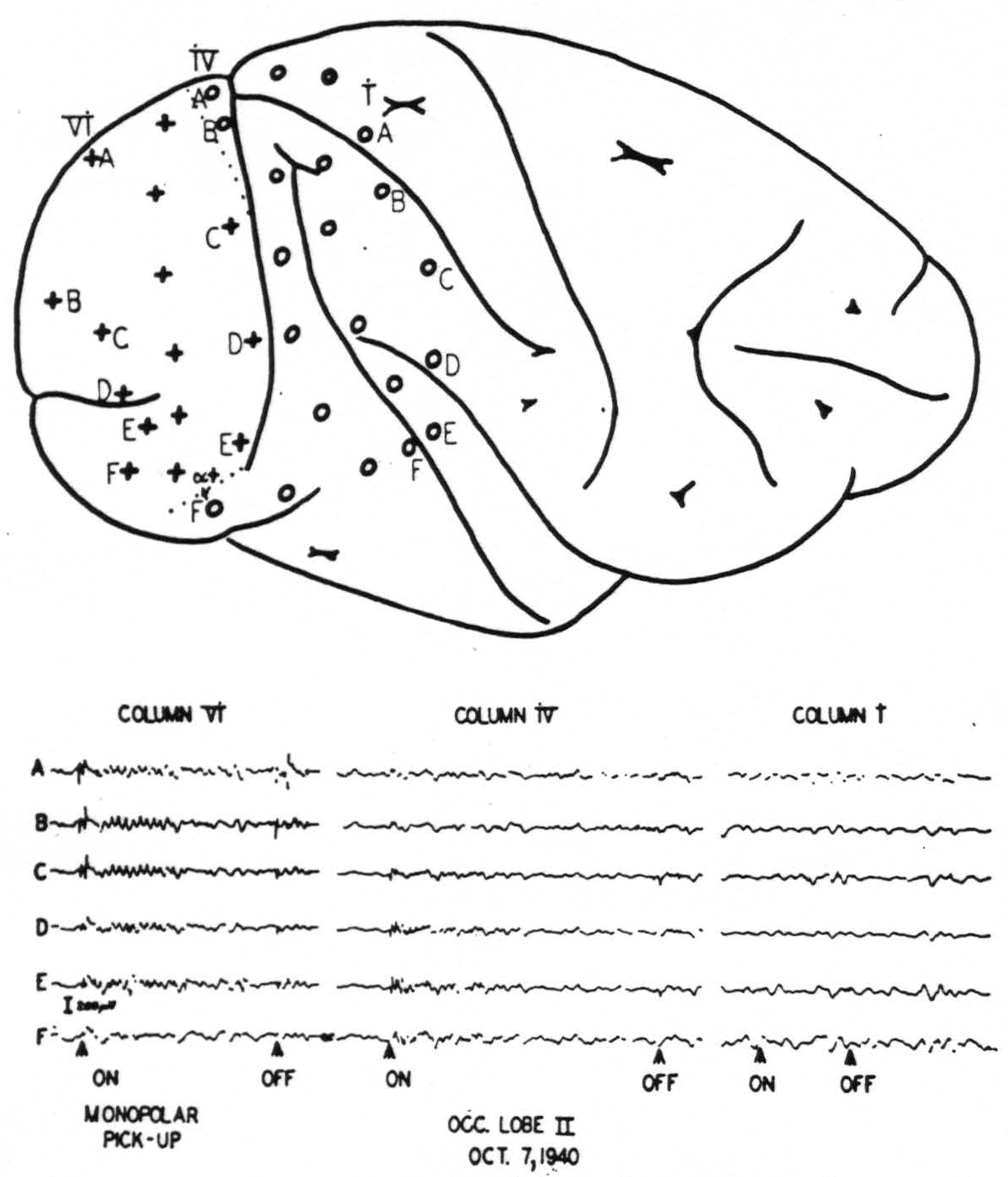
Figure 1. Upper diagram: position of electrodes on cortex. Lower graph: Records from the electrodes indicated. Note “on” and “off” effects and subsequent ripples in electrodes on striate area. Compare also Fig. 2.
the hemisphere about 3 mm. behind the parieto-occipital incisure. It runs then parallel to, and within about 1 mm. of, the largest part of the sulcus simiarum to swing ventrally backwards at a distance of about 6 mm. from the sulcus occipitalis inferior. Histological examination showed this area to be identical with area 17. It is interesting to see how precisely the “on” and “off” effects were restricted to the striate area in the monkey. Fig. 2 shows in a microphotograph the locus of Electrode B (column IV) given diagrammatically in Fig. 1. It was situated just beyond the striate area, within the “margo magnocellularis”

Figure 2. Posterior lip of lunate sulcus. Place of electrode B of row iv of fig. 1 is indicated by the lesion. It is about 0.5 mm in front of the anterior boundary of the striate area, just over “OBγ.” No prompt response to light was observed from this locus.
of 18 and did not give any primary response to light. In the chimpanzee, the striate area bears a relationship to the lunate sulcus similar to that prevailing in the macaque. However, as indicated by Campbell (2) and by Brodmann (18), the ventral boundary of the striate area is much higher in the anthropoid ape than in the monkey.
We have made no attempt to study the internal organization of area 17. Under Dial anesthesia strychninization on a given point of area 17 (Fig. 3) causes spikes to appear within the striate area only in the immediate vicinity of that point and not elsewhere in area 17. This fact had been demonstrated by Dusser de Barenne and McCulloch (19) and is amply confirmed here. However, after strychninization of 17 spikes do appear within area 18, but not within area 19. Finally, a point of particular interest, strychnine applied to area 17 never
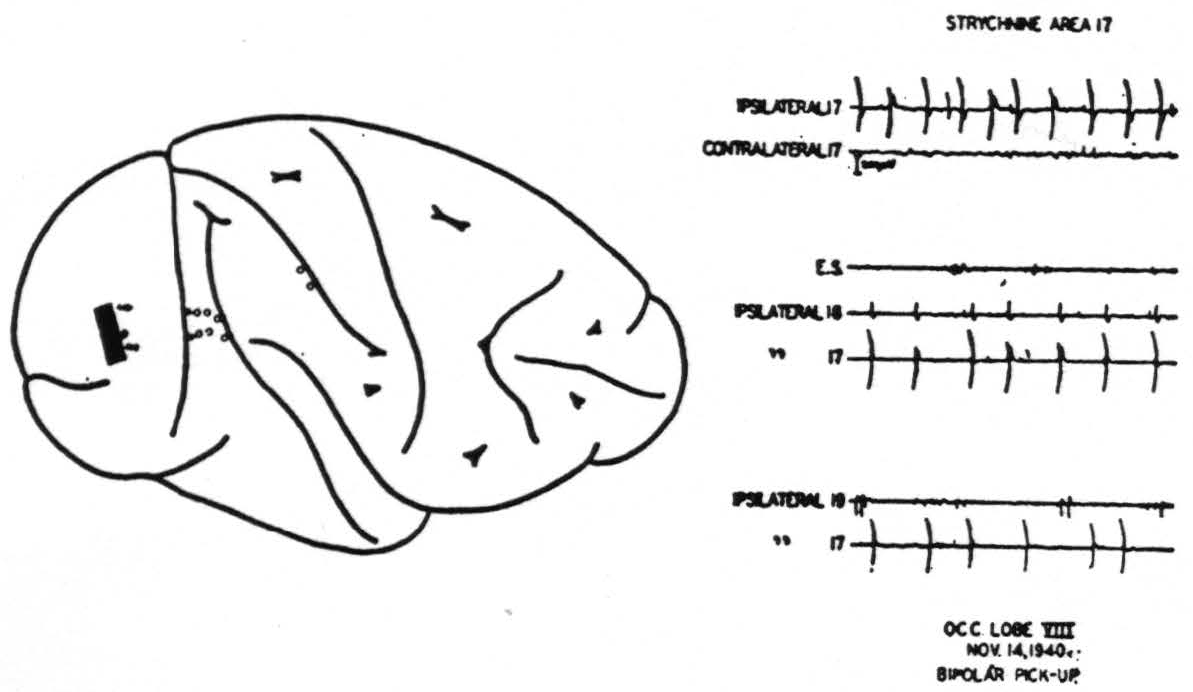
Figure 3. Result of strychniniration of area 17. Locus of strychninization and of pick-up electrodes indicated on the diagram. E. S.: Elliot Smith's visuo-sensory band β (cf. text).
fires into the contralateral hemisphere, not even into the contralateral area 17 (Fig. 3).
In all these respects area 18 is in sharp contrast to 17 (Fig. 4). The prompt “on” and “off” effects seen in 17 are represented here only by belated ripples of approximately a frequency, say 7 per second. Local strychninization of 18 results in widespread “firing” within 18 itself, even from the medial to the lateral and from the lateral to the medial aspect of the hemisphere.
Area 18 fires also in area 17, but the firing into 17 appears to occur only into a small roughly wedge-shaped area with its base in the neighborhood of that point of 18 which is being strychninized.
Area 18 fires area 19 and the firing extends into a narrow band on the ventral or dorsal lip of the sulcus intra-parietalis. This is that part of the cortex which was described by Elliot Smith (7) as the “visuosensory band β."
Moreover, area 18 fires into the cortex covering a part of the lower margin of the second and into the entire length of the third temporal convolutions (Fig. 5). In their turn, these areas fire only locally, and not into 18.
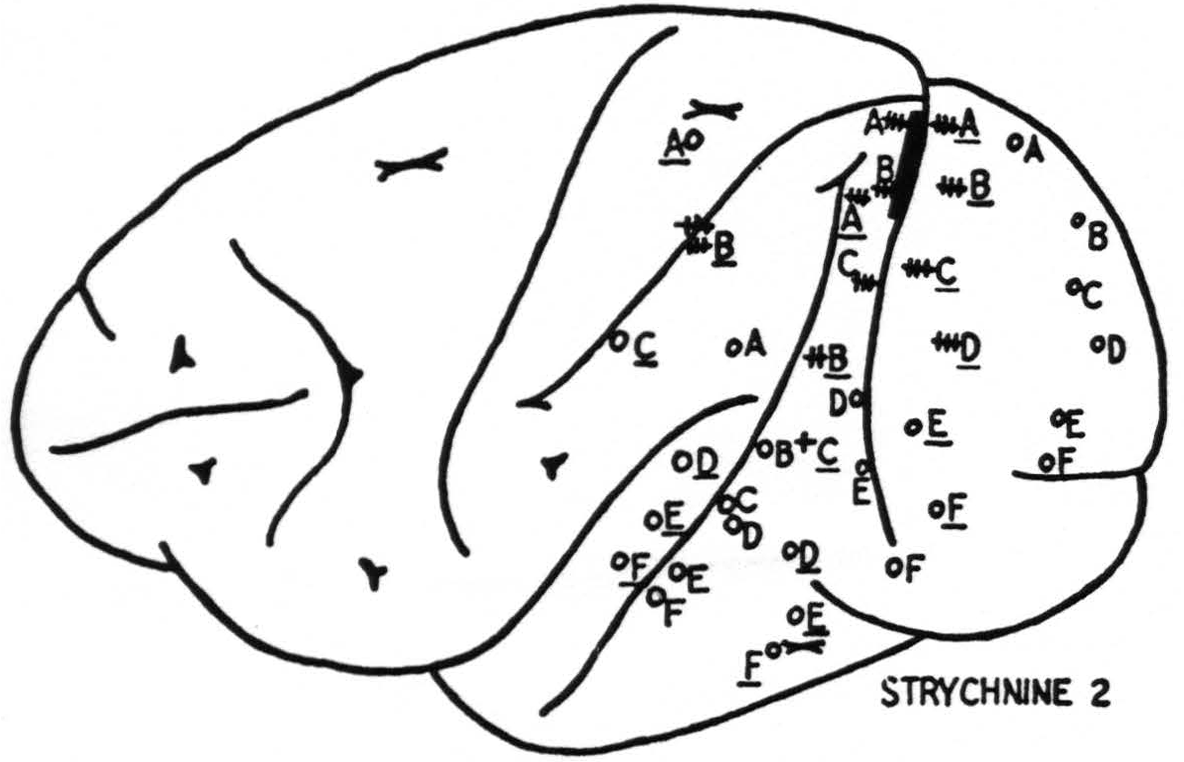
Figure 4. Results of strychninization of area 18. +++: very large, ++: large, +: moderate strychnine spikes, o: no spikes from strychinization.
Finally, strychninization of area 18 leads to firing of symmetrical foci of the opposite hemispheres (Fig. 9). We find clear evidence for callosal connections only between the two areas 18. We have not observed 18 to fire into the contralateral 17 or 19.
Within area 19 strychnine fires the cortex only in the immediate vicinity of the locus of application and initiates a suppression of electrical activity which begins in regions close to the strychninization and sweeps across the entire hemisphere (Fig. 6). There is no evidence of callosal connections nor of any other cortico-cortical connections originating in area 19 (Fig. 9).
Discussion.
A. Cortical Areas.—The number of fields differentiated by physiological neuronography in the occipital lobe is the same as that which Brodmann, Mauss and Elliot Smith propounded and upon which C. and 0. Vogt and v. Economo and Koskinas built their further elaborations. It bears no relation to the scheme set forth by Beck. The boundary between 17 and 18 found by histological observations is confirmed by physiological methods.
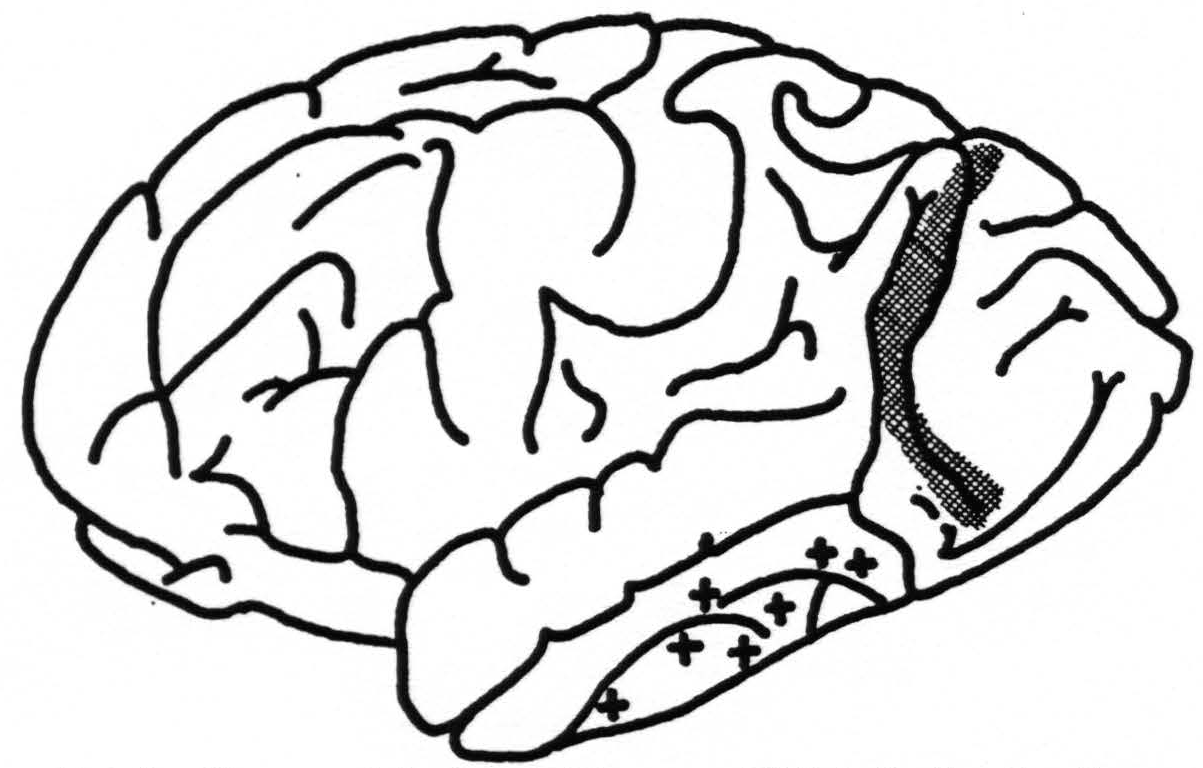
Figure 5. Diagram of brain of chimpanzee XXI indicating location of strychninizations in occipital lobe (cross-hatched area) which fired into the temporal lobe at points indicated by +.
Area 18, for which Elliot Smith's term parastriate area appears advisable, is certainly larger than indicated by Mauss, and perhaps even larger than shown by Brodmann. It extends both in the macaque and the chimpanzee beyond the main part of the sulcus lunatus, in the former very often even beyond the crest of the angular gyrus (to use Mettler's (20) nomenclature for the macaque). It includes, in other words, a part of the cortex which many students of cytoarchitecture regarded as 19. It does not show exactly the same cytoarchitecture throughout. Fig. 7 shows the deepest portion of the
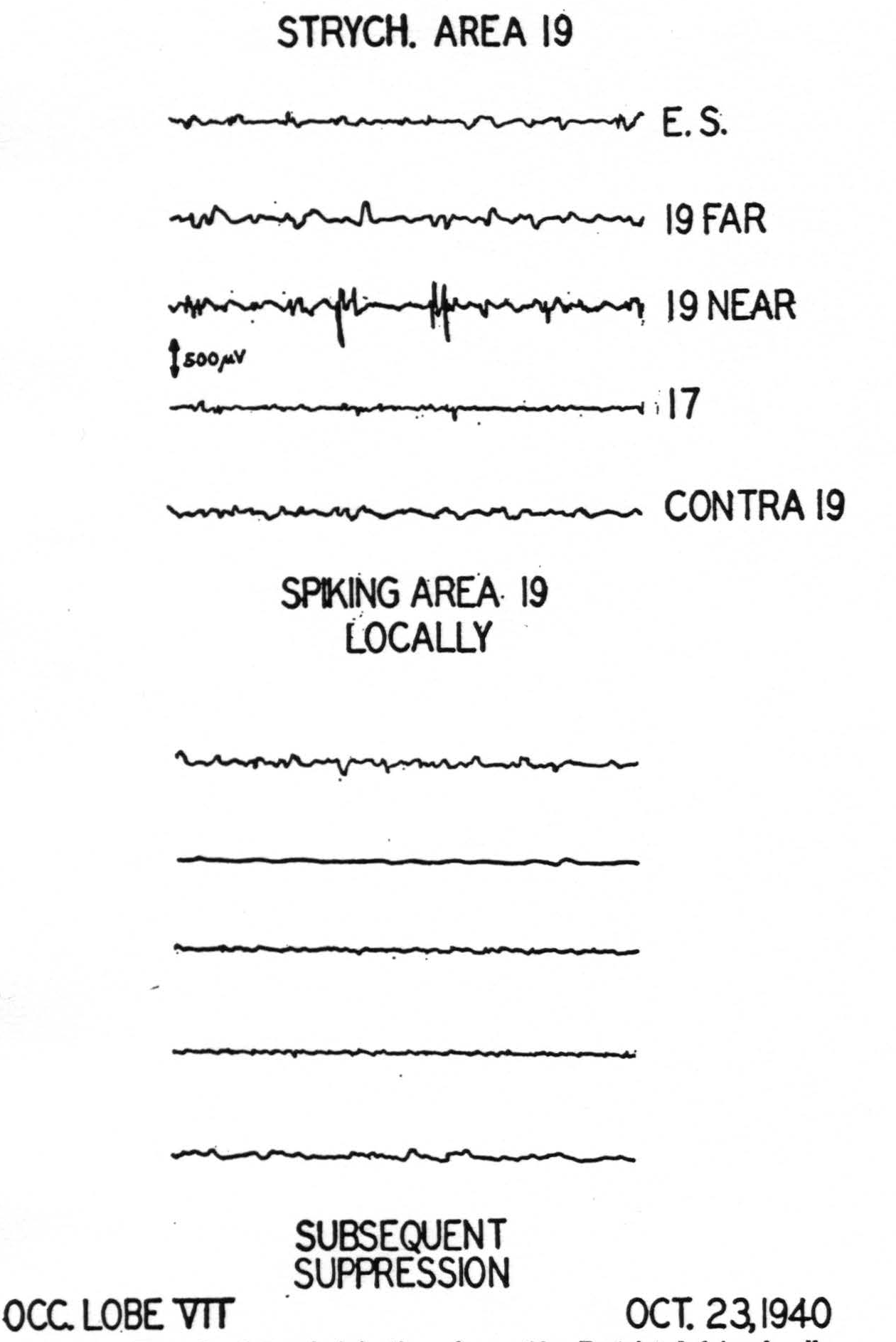
Figure 6. Records of strychninization of area 19. Restricted firing locally, subsequent suppression of electrical activity of cortex.
lunate sulcus in the macaque. It is easily seen that the cortices on the two lips of that sulcus show enough cyto-architectural differences to warrant the establishment of two areas corresponding roughly to Beck's (16) “opal” and “oprl.” Yet the frontal part, i.e., that part which is further away from the striate area, shows the
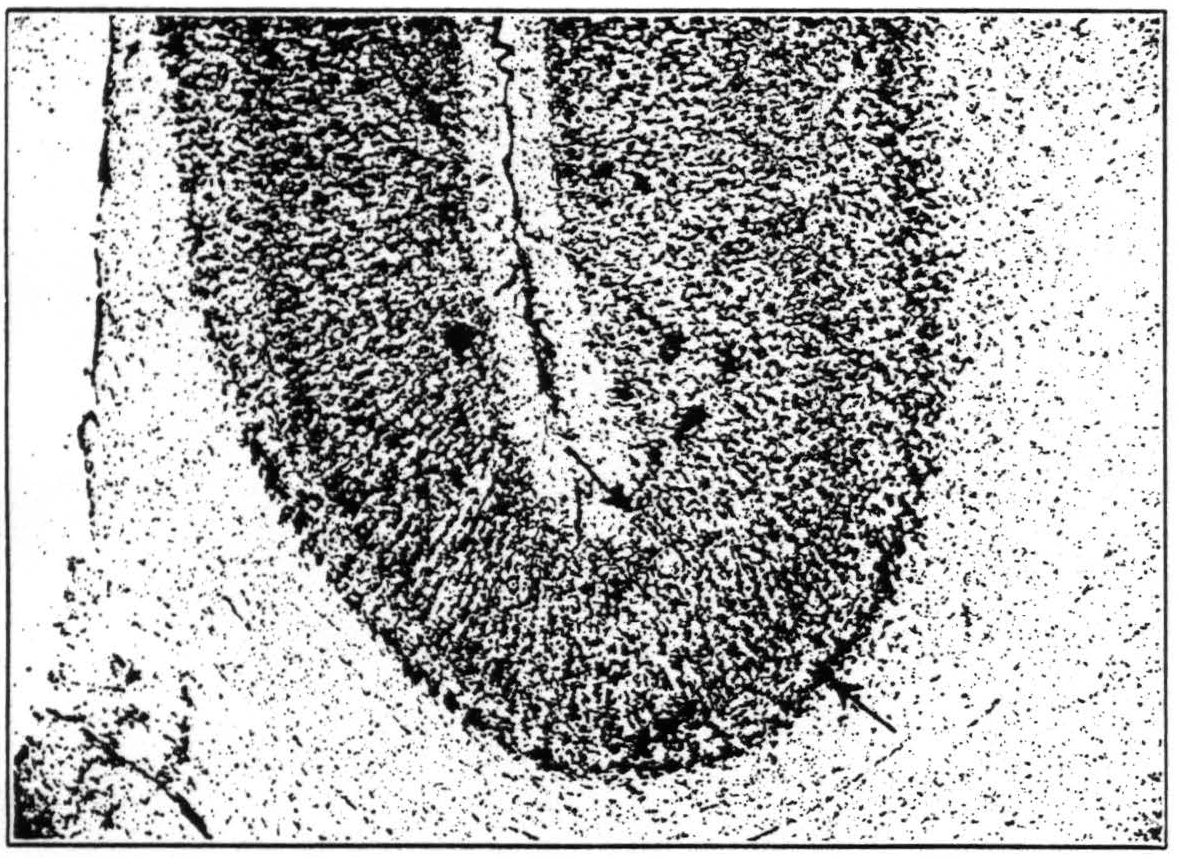
Figure 7. Cortex in the depth of the lunate sulcus, occipital to the left of the reader. The structure of the cortex changes continuously through the region indicated by arrows. Note the sharp boundary between cortex and medulla to the left, the blurring of this boundary to the right. Note also the difference in the relative breadth of layers IV and V, in cell-density and cell size in layer III.
physiological properties of 18, and there is no reason to split off the caudal part of 18, as Beck evidently does, and create a new area with undefined functional properties. Strychninization of “opr” fires “opa” on the medial side of the hemisphere and near the dorsal margin, i.e., wherever this formation is accessible. Physiological neuronography has to consider these two areas as one unit.
Area 19 is functionally akin to 3 other cortical areas, 8s, 4s and 2s wherein strychninization has a similar suppressor effect. Each of these other suppressor areas has been shown (21) to produce this phenomenon by virtue of connections with the nucleus caudatus. Therefore, it seems reasonable to expect that the chief projections of area 19 also go to some deeper structure, perhaps even to the same caudate nucleus. Be this as it may, there is evidence that area 19 is concerned with vision only as recipient of impulses from area 18, which is a recipient of those from 17. To emphasize this indirect connections as well as the fact that area 19 has functional relations with all parts of the cortex, we propose for it Brodmann's term “preoccipital” area.
The preoccipital area, as revealed by the method of local strychninization, cannot clearly be characterized by its cytoarchitecture. The subtle differences between OB and OA, i.e., between 18 and 19 described by v. Economo and Koskinas for the human brain cannot be identified in the brain of the chimpanzee, and still less in that of the macaque. Several macaque brains in which the pre-occipital area had been mapped out physiologically and marked on the surface have been histologically examined. The second and third layer may be more clearly marked off from each other in the preoccipital than in the para-striate area. The third layer may not be quite as broad in the former as in the latter, and the difference in cell size between upper and lower tiers of III is perhaps less striking in the preoccipital than in the parastriate area. The fourth layer may not be quite as dense in the former area as in the latter. Moreover, the parastriate shows not infrequently large cells in IV (border pyramids of O'Leary (22) or large star pyramids of Lorente de No (23) ?). These cells which are quite conspicuous have not been seen in the preoccipital area. The fifth layer, a rather light band in both areas, may contain slightly larger cells in the preoccipital area. Occasional large cells are undoubtedly present. The sixth layer has much the same appearance in both areas. The boundary of VI against the white matter is probably less sharp in the preoccipital than in the parastriate area. But when all this has been said there remains the uncomfortable feeling that it is well nigh impossible to indicate sharply and clearly the boundary between the two areas under the microscope.
Additional support for the contention that this boundary is evasive may be found in Filimonoff's data (cf. page 166). The table given there shows that the variabilities increase by leaps and bounds as one goes from 17 over 18 to 19. It is almost impossible to miss the boundary between 17 and 18, and the coefficient of variation of about 5 found for the striate area may be accepted as a good indication of the “true” variability of a cortical area. The significantly higher variability of 18 and the still higher one of 19 may possibly be due to just this —that the boundaries of these areas are much more difficult to recognize. In view of the large sampling errors it may be merely a coincidence, but it is striking that the variability of 19 which has two ill-defined boundaries is about twice as high as that of 18 which has one ill-defined and one well-defined boundary.
Preoccipital and parastriate area differ more by their connections than by their cytoarchitecture. Association fibers from the striate area reach the parastriate but not the preoccipital area. Callosal fibers are present in the former but not in the latter, and the projective systems which have not been investigated in this study, almost certainly differ in their anatomy, for they certainly differ physiologically.
There can be little doubt that Elliot Smith's “visuo-sensory band β” is a continuation of area 19s. He himself considered it as a connections between his “peri-striate” and “somesthetic” areas. Brodmann (6) stated for the human brain that the cortex within the intraparietal sulcus was of a structure similar to that of “19.” v. Economo and Koskinas described that cortex as resembling their “PD,” a formation on the anterior wall of the postcentral sulcus. Neither Brodmann (6) nor Mauss (8) mention it for the cercopithecus (which, for them, included the macaque), but v. Bonin (24) described a visuosensory band β in the brain of the cebus. Physiological observations leave no doubt about the existence of Elliot Smith's band β in the macaque and the chimpanzee. They serve once more to show the essential similarity of the structure of all primate brains. From the histological appearance of the cortex on either wall of the intraparietal sulcus one would expect this cortex also to be fired because it resembles area 19. The observed firing on either dorsal or ventral lip of the sulcus would then easily be accounted for by a small shift of the entire area in its position within the sulcus. McCulloch and Garol (25) observed suppression of electrical activity of the cortex after strychninization of the lip of the intraparietal sulcus just as after strychninization of that suppressor area which they called 19s. The fact that histologically as well as physiologically the cortex on the lip of the intraparietal sulcus, i.e., the visuo-sensory band, and the preoccipital area share the same properties, justifies considering both as parts of the same entity.
We are therefore not prepared to accept the boundaries of 18 and 19 for the human brain which have been indicated by Brodmann or by any of his successors, and we doubt whether histology alone is able to yield trustworthy results. We would, in fact, suggest that their associative, radiative (corticopetal) and projective (cortico-fugal) systems be studied as a possible basis for their distinction.
B. Association Bundles.—Our observations throw some light on the question of association bundles of the occipital lobe which has agitated neurologists for a long time. The existence of long association fibers in the white matter of the hemisphere is undisputed. Two problems concerning them arise, however. The first question is this : Do these fibers form compact bundles or are they arranged in a more diffuse way within the medulla? . The reverse of this question constitutes also a legitimate problem : Are those tracts which can be demonstrated within the medulla composed of association fibers all running between the same two endpoints? The second question is of a more physiological nature : Granted the existence of association tracts, do their fibers conduct in one way, say only from point A to point B, or do they conduct both from A to B as well as from B to A?
It happens that the first type of problem is well exemplified by the association bundles of the occipital lobe. As is well known, four bundles have been described (see Fig. 8) : (1) Sachs’ (26) fasciculus transversus cunei, (2) Vialet's (27) fasciculus transversus lingualis, (3) the stratum calcarinum, and (4) Wernicke's (28) vertical occipital fascicle. The first three of these are easily seen in all primate brains. They course over or under the posterior horn of the ventricle, or around the depth of the calcarine fissure, respectively. In all these places they pass through “narrows” of the white matter and are therefore squeezed together into compact bundles.
Wernicke's vertical bundle, running lateral of the ventricle, is well seen in the macaque wherein it was first recognized by Wernicke himself, but cannot be easily demonstrated in the human brain, as both v. Monakow (29) and Dejerine (30) seem to agree. Only Redlich (31) labelled it in a section of a human brain. An examination of the macaque's brain revealed clearly Wernicke's bundle, but its existence could not be ascertained in sections of the brains of chimpanzee or man. The reason for this difference may not be far to seek: there is much more room in the spacious white core of the occipital lobes of chimpanzee and man than in the sparse white matter of the macaque's occipital lobe. Hence the association fibers which are compressed into a compact
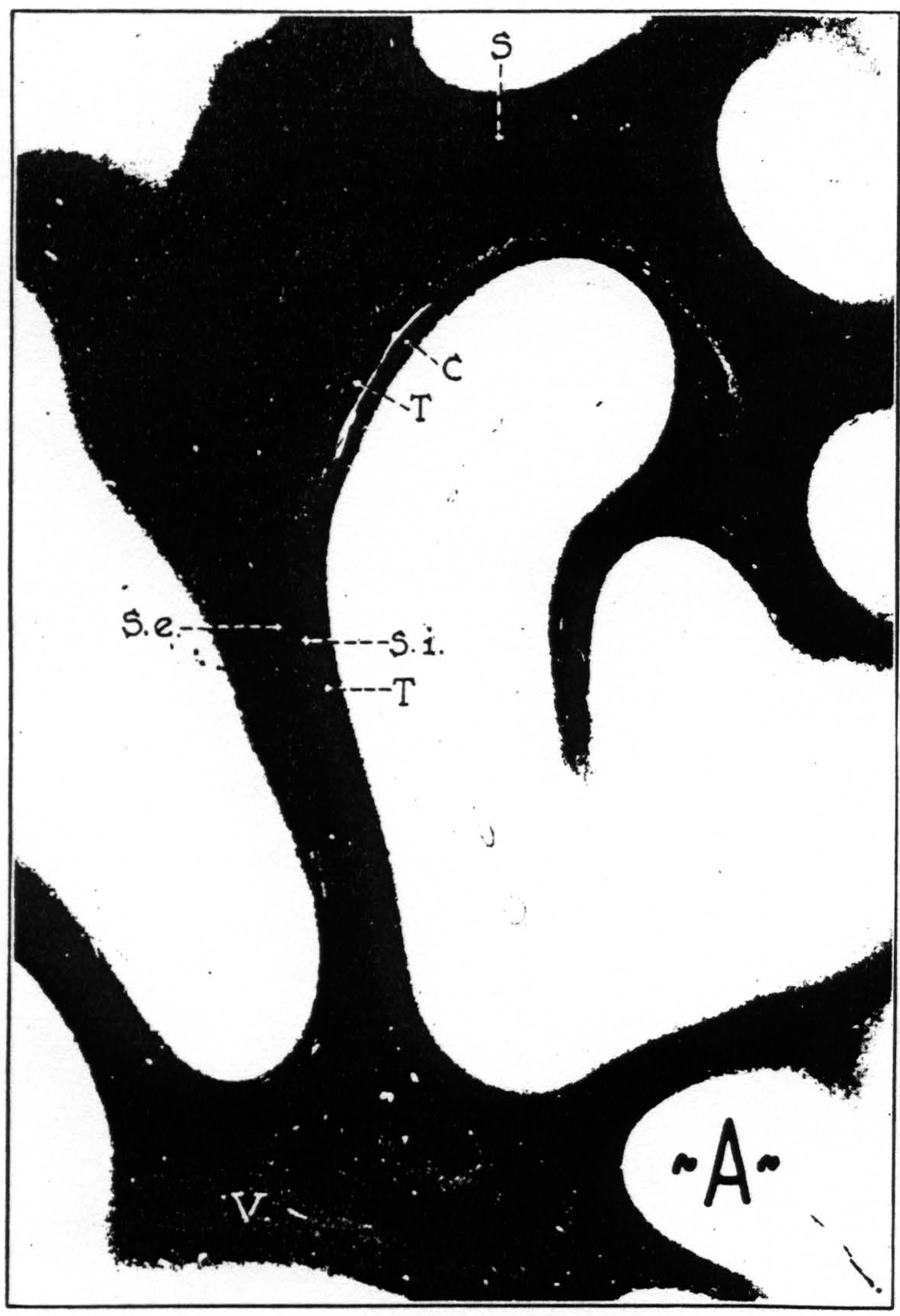
Figure 8a. Transverse section through brain of chimpanzee, stained after Weigert. C, stratum calcinarum; S, fasciculus transversus cunei; S. e., stratum sagittale externum ; S. i., stratum sagittale internum; T, tapetum; V, fasciculus transversus lingualis.
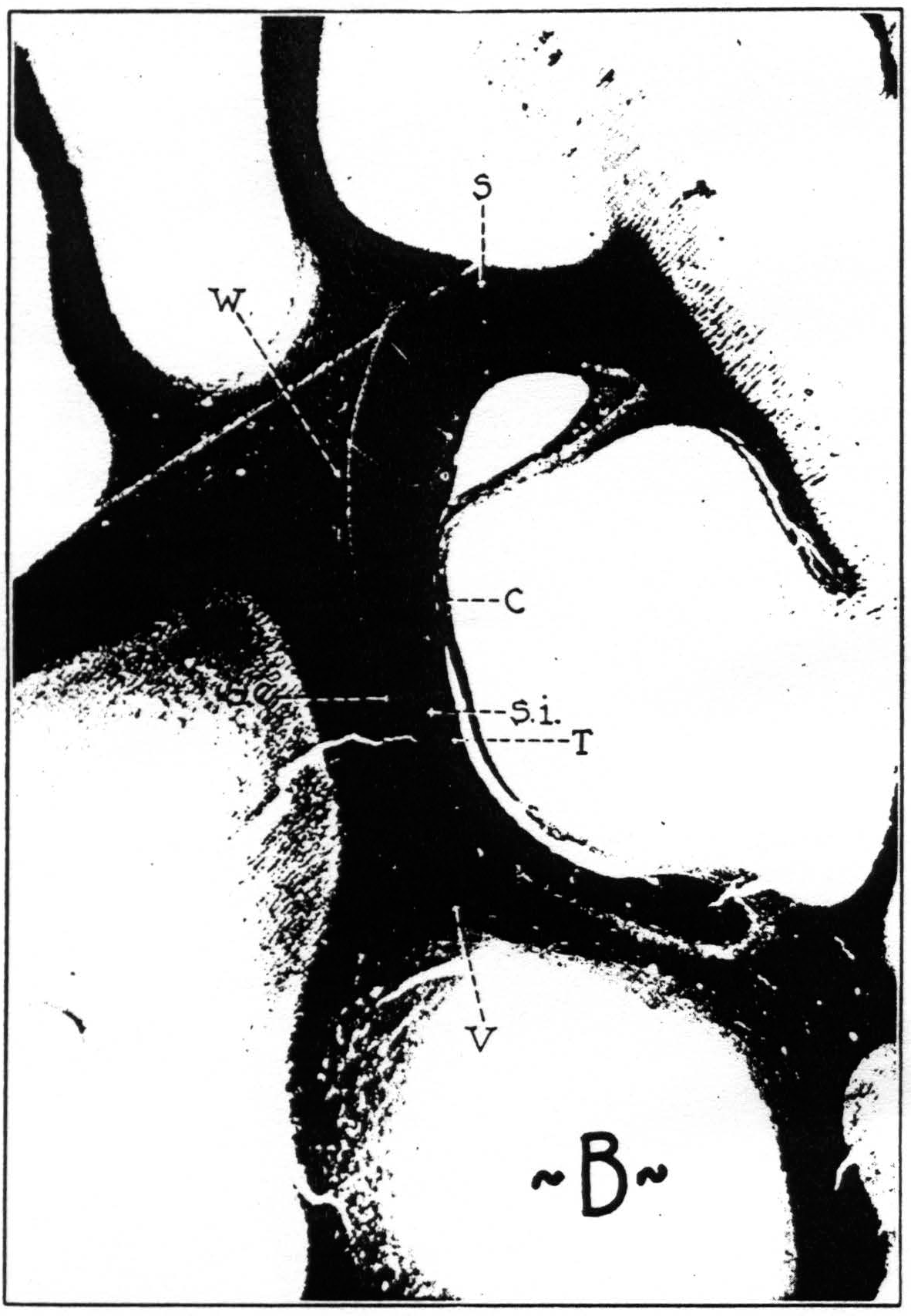
Figure 8b. Transverse section through brain of macaque, stained after Weigert. C, stratum calcinarum; S, fasciculus transversus cunei; S. e., stratum sagittale externum; S. i., stratum sagittale internum; T, tapetum; V, fasciculus transversus lingualis; W, vertical occipital fasciculus.
bundle in,the macaque, may be more diffusely spread out over a larger space in the higher primates.
All four systems can be looked upon as subserving associations within the parastriate area. The widespread firing observed within this area supports this view. It can be considered as an additional proof of their existence and can moreover be taken as evidence that these bundles conduct in both directions.
Questions of both the first and second type concerning the inferior longitudinal bundle are raised by the firing into the temporal lobe from the parastriate area. This tract, first described more than a century ago by Reil (32) and Burdach (33) has been frequently under discussion, and many authors doubted whether it was an association bundle at all. An anatomically well-defined bundle connecting temporal and occipital lobe has been demonstrated in gross preparations by many authors. Hultkrantz (34) and Rosett (35), the former of whom gives an instructive photograph of a dissection, may be mentioned among modern writers. It was first thought that within the occipital lobe this bundle ran in the stratum sagittale externum. But when Flechsig(36), Pfeifer (37), Niessl von Mayendorf (38) and several other authors found by myelogenetic and pathological studies that the optic radiation coursed in the external sagittal stratum—a fact confirmed for the macaque by Polyak (39) among others—it was frequently denied that there existed any fibers forming an inferior longitudinal bundle. One of the latest authors to write on this question, Brouwer (40), inclines to the belief, however, that there are indeed fibers in the external stratum belonging to an inferior longitudinal fascicle. It may not be amiss to point out that the anatomy of this region differs so much in the macaque, chimpanzee and man that it does not seem justified to draw inferences regarding man from experimental observations. We possess a study of the comparative anatomy of this bundle by Redlich (31) which contains figures for man, hylobates and macaca, aside from a host of other animals, including many primates. To illustrate this problem of comparative neurology still further, figures 8 A and B give comparable transverse sections through the brains of macaque and chimpanzee. For man, numerous figures are available. Brouwer's photograph (40) and Déjírine's drawing (30) may be recommended. By human standards the internal stratum sagittale of the monkey is diminutive. Since the optic radiation is of about the same relative size in all primates, it is clear that the composition of external and internal stratum must be different in man and monkey. There seems little doubt that certainly in the monkey, and probably in man, too, the stratum sagittale externum contains fibers of the optic radiation, but that does not exclude the possibility that this stratum also contains fibers which form an inferior longitudinal bundle. The results of strychninization of the parastriate area speak strongly in favor of long association fibers between this area and the third temporal convolution. They indicate, moreover, that this system of fibers conducts only in an occipito-temporal direction, not from the temporal to the occipital lobe. The experimental results suggest moreover that there are fibers passing from the dorsal part of the parastriate area to the temporal lobe. These may run first in the vertical occipital bundle of Wernicke, to sweep over into the inferior horizontal fasciculus in a ventral level. It should not be too difficult to test this assumption.
That the inferior longitudinal fasciculus conducts impulses only one way is not refuted by an observation of Edinger (41) who found, in a human brain, degeneration in the inferior longitudinal fasciculus after a lesion of the temporal lobe, for the degenerated fibers which he saw may have belonged to the optic radiation. Their looping course in the temporal lobe is well known. Bucy and Klüver (42) similarly mention degeneration of temporo-occipital fibers after temporal lobectomy in the macaque. These, however, may well have been retrograde degenerations, since they examined their animal two years after the temporal lobes had been removed.
C. Callosal Connections.—The question of callosal connections between the occipital lobes has also puzzled anatomists for some time. We do not intend to review the older literature, particularly since this has recently been done by Mingazzini (43). Suffice it to remind the reader that van Valkenburg (44) by means of Nissl and Pal-Weigert preparations came to the conclusion that “one will have to reckon with the fact that the area striata has no commissural connection.” He arrived at this result after studying experimental sections of the corpus callosum in mice, rabbits and cats and after investigating a number of pathological cases (three of which had lesions involving the occipital connections). van Valkenburg's result did not go undisputed. de Villaverde (45) investigated the callosal connections of the rabbit by means of Marchi's method. He made small lesions in one hemisphere and traced the degenerated fibers to the other hemisphere. “Contrary to what has been affirmed, area 17 of Brodmann contains an abundant system of callosal fibers.” Whether these were homotopic or heterotopic, the author was not prepared to state definitely. Polyak (46) also using “Marchi's method found callosal connections between the two striate areas in the brain of the cat. Biemond (47) who worked on the macaque (Java monkey) paid more regard to the subordinate organization of the callosal fibers than to their point of origin. Mettler (48) investigated the brain of the macaque by means of Marchi's method. He observed callosal connections arising from the macular part of the striate area, but leaves it open whether the extramacular part, too, contributes to the corpus callosum. Furthermore, he saw callosal fibers coming from 18 and 19 going to the striate area of the opposite side. Mettler also reviews critically much of the literature. Sunderland (49) studying also the macaque concerned himself principally with the arrangement of the fibers within the corpus callosum. In one brain (experiment 4) he placed a lesion in 17 and the adjacent part of 18, and found abundant degenerated fibers in the splenium. Whether they came from 17 or 18 he does not decide. Pines and Maiman (50) appear to be the only ones who recently investigated the origin of callosal fibers by studying retrograde cell degeneration after cutting the corpus callosum. They investigated the motor and frontal regions of dogs, and report briefly on observations in pathological human brains. The areas possessing callosal connections are enumerated. Of the occipital lobe only 19 is mentioned. This may well be a part of the cortex, however, which in reality has parastriate properties.
Physiological methods of investigating the pattern of callosal connections in the monkey have recently been employed by Curtis (51) who came to results regarding 18 and 17 in essential agreement with our own. The method of physiological neuronography was applied to the cortex of the macaque by McCulloch and Garol (25) and to that of the chimpanzee by Bailey, Garol and McCulloch (52).
The apparent discrepancy between anatomy and physiology may partly be accounted for by the fact that most histological investigations were carried out on non-primates. Apart from Biemond, Mettler and Sunderland are the only ones who worked on macaques. Sunderland's results indicate the probable existence of connections between the striate areas rather than their absence, but they are by no means conclusive. Mettler is the only author who definitely asserts in the monkey the presence of callosal fibers between the two striate areas. As a further contribution to this problem, we have prepared, in collaboration with Dr. Percival Bailey, some monkeys for the study of retrograde degeneration in the cortex after section of the corpus callosum. Only one macaque could be examined so far and even that one only in a preliminary way. The animals were killed 16 days after the operation, and serial transverse sections of the whole brain were prepared. A casual inspection revealed the presence of degenerated cells in the III, V and VI layer of area 18, and in the VI layer of area 17. The results for 18 agree with Pines’ and Maiman's results in the dog as far as layers are concerned. The presence of degenerated cells in the striate area is disturbing, but not necessarily in contradiction to results obtained by physiological methods. For recognizable electrical disturbances depend upon the synchronous firing of a sufficient number of cells whose axons remain sufficiently concentrated to produce a voltage hundreds of times larger than could be expected from but a few cells going off together, and it is not proved that the requisite concentration of cells exists in the striate area. Moreover, it has never been shown that the axons of the “callosal” cells of the striate area go to homologous loci of the other hemisphere, or even to any place of the contralateral cortex. Examples of “heterotopic” crossed firing have frequently been observed. The most impressive instance is probably that recorded by Bailey, Garol and McCulloch (52). They observed crossed firing in the chimpanzee from band II of one hemisphere to the contralateral parastriate area.
While the problem of corpus callosum and striate area is admittedly not yet solved, the fact that local strychninization under Dial narcosis fails to reveal any such connection can be regarded as definitely established.
D. Clinical Application.—It is naturally tempting to apply our results to man and to clinical pathology. To extrapolate from macaque and chimpanzee to the human brain would have no more value than that of a heuristic hypothesis. Since the functional organization is essentially identical in both primates, one might assume that that is also true for man, in spite of his much larger parastriate (and preoccipital?) area. Nevertheless, the pre-occipital suppressor area can be diagnosed by electrical stimulation on the operating table, and the histological diagnosis of the striate-parastriate boundary is one of the easiest tasks of cytoarchitectonics. It should not be long, therefore, before we have, concerning man, information about the extent of the parastriate area as defined on the foregoing pages. There are hints in Foerster's (53) observations on the effects of stimulation of the occipital lobe that much of what he calls “19” corresponds to our parastriate area, since stimulation of these two areas elicited the same kind of sensations. Stimulation of “18” and “ 19 “ gave rise to optic sensations of richer content and greater complexity than stimulation of 17. Close to 17, the electrodes caused flames or blue clouds to approach the patient, but when it was applied further frontally, a friend waved his hand to him, or a butterfly fluttered toward him. Upon stimulating “19,” birds were flying toward one patient. “Suddenly he stopped describing his visions and lost consciousness.” Is this due to a stimulation of the preoccipital suppressor area?
The goal of mapping out the occipital areas may appear as a rather dry and pedestrian affair, yet one should hesitate to probe much deeper into the secrets of vision from the basis of such facts as are given on the preceding pages. Even to apply our newly won knowledge to clinical problems of the disturbances of the higher visual functions is fraught with grave difficulties. These disturbances involve frequently, although not always, parts of the cortex which lie outside of the occipital lobe. It is precisely there, in the temporal and the parietal lobes, that far reaching evolutionary changes have occurred during primate phylogenesis, i.e., during anthropogenesis. In an elementary way, this was illustrated by a recent encephalometric study (54). To argue from monkey or chimpanzee to man is in this instance therefore clearly not permissible. It should also be kept in mind that physiological neuronography does no more than reveal the presence of certain pathways and that
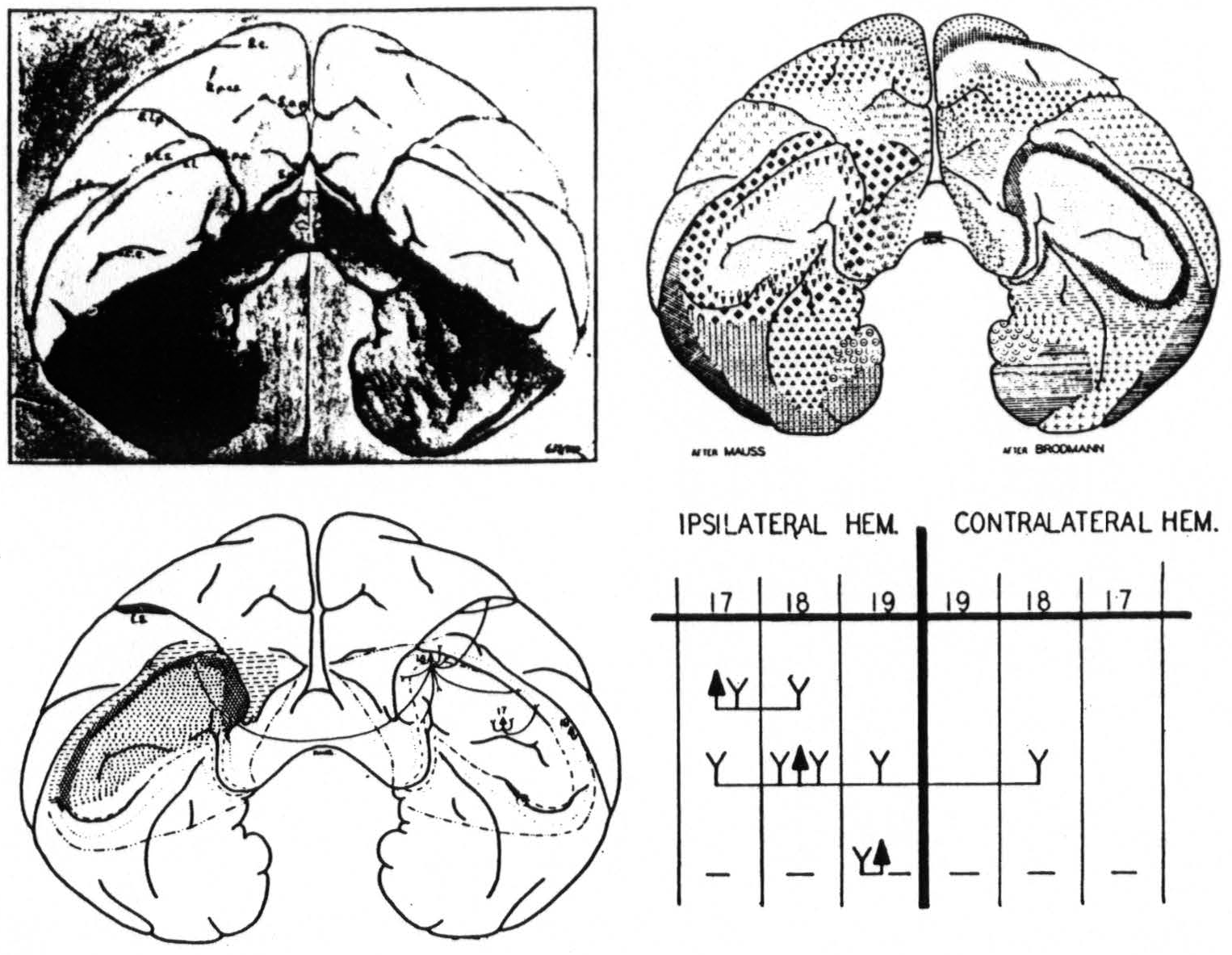
Figure 9. Equiareal projection of the occipital poles of the brain of the macaque. Upper right indicates the positions of Brodmann's and Mauss' areas, lower left indicates the areas found by physiological neuronography, lower right gives firing diagram: ▲: arcus strychninized, Y: areas fired, —: areas suppressed.
we have no ground to think of cortical events as occurring only along these pathways and no others. Neuronography tells us, moreover, directly nothing about those cortical events which, as we, assume, are the physiological parallel to mental processes. That very assumption, however, is a philosophical problem about which even experts may have different opinions. In fine, an attempt to enter the realm of the mind or to discuss how it is perturbed by cerebral lesions needs much more thorough preparations and much firmer foundations than have or can be given within the limits of this contribution.
To sum up (Fig. 9) : The occipital lobe of macaque and chimpanzee bears three functionally distinct areas for which the names striate, parastriate and preoccipital are proposed.
- Strychninization of the striate area leads only to local firing within that area and to firing of the para-striate area. Visual stimuli lead to prompt and large “on” and “off” effects in its electrocorticogram. It is well characterized by its histological structure from which its name is derived.
- Strychninization of the parastriate area leads to widespread firing within this area, to firing into a circumscribed part of the striate area in the vicinity of the point of strychninization, to firing of the preoccipital area and to firing of the cortex on the inferior temporal and part of the middle temporal convolution. Strychnine spikes also appear within the parastriate area of the opposite hemisphere. Visual stimuli lead in the parastriate area only to the appearance of belated “ripples.” Its anterior margin is histologically ill-defined, and lies anterior to the position given on cytoarchitectonic maps.
- In the preoccipital area strychninization leads to only local firing restricted to the same hemisphere. Its strychninization leads, however, to a suppression of the electrical activity of the cortex which spreads slowly over both ipsi- and contralateral hemisphere. It is histologically ill-defined, but there is good reason to assume that it extends into the intraparietal sulcus and that it is there identical with the cortex described as the “ visuosensory band β.”
Footnotes
Literature Cited
Munk, H. 1886. *Sitz. Ber. Kgl. Preuss Akad. d. Wiss. Math. Physik. Klasse. *(Cited after Brouwer.)
Campbell, A. W. 1905. “Histological Studies on the Localization of Cerebral Function.” Cambridge: University Press.
Fulton, J. F. 1937. Bull. Hist. Med., 5: 895.
Meynert, Th. 1868. “Der Bau der Grosshirnrinde und seine örtlichen Verschiedenheiten.” Leipzig.
Bolton, J. S. 1900. Phil. Trans. B. Soc. London, ser. B., 193: 165.
Brodmann, K. 1909. “Vergleichende Lokalisationslehre der Grosshirnrinde.” Leipzig: J. A. Barth.
Smith, G. Elliot. 1907. J. Anat. & Physiol., 41: 237.
Mauss, T. 1908. J. f. Psychol, u. Neurol., 13: 263.
Mauss, T. 1912. J. f. Psychol, u. Neurol., 18: 410.
Vogt, C. and O. 1919. J. f. Psychol, u. Neurol., 25: 279.
v. Economo, C., and G. N. Koskinas. 1925. “Cytoarchitecktonik der Grosshirnrinde des erwachsenen Menschen.” Berlin u. Wien: J. Springer.
Kleist, K. 1934. “Gehirnpathologie.” Leipzig: J. A. Barth.
Lange, J. 1937. Ztschr. f. d. ges. Neurol, u. Psychiat., 158: 247.
Filimonoff, N. I. (1932) J. f. Psychol, u. Neurol., 44: 1.
Filimonoff, N. I. (1933) J. f. Psychol, u. Neurol., 45: 69.
Beck, E. 1934. J. f. Psychol, u. Neurol., 46: 194.
Ngowyang, G. 1934. J. f. Psychol, u. Neurol., 46: 351.
Brodmann, K. 1912. Ant. Anz., 41, Erg.-H. 7, S. 157.
Dusser de Barenne, J. G., and W. S. McCulloch. 1938. J. Neurophysiol., 1: 69.
Mettler, F. A. 1933 Am. J. Phys. Anthropol., 17: 309.
Dusser de Barenne, J. G., and W. S. McCulloch. 1941. J. Neurophysiol., 4: 311.
O'Leary, J. L. 1941. J. Comp. Neurol., 75: 131.
Lorente de Nó, B. 1938. “The Cerebral Cortex.” In : Fulton, J. F., “Physiology of the Nervous System.” London, New York, Toronto: Oxford University Press.
v. Bonin, G. 1938. J. Comp. Neurol., 69: 181.
McCulloch, W. S., and H. W. Garol. 1941. J. Neurophysiol., 4: 555.
Sachs, H. 1892. “Das Hemisphärenmark des menschlichen Grosshirns.” Leipzig: G. Thieme.
Vialet, 1893. Compt. rend. Soc. de biol., 45: 793.
Wernicke, C. 1881. “Lehrbuch der Gehirnkrankheiten.” Leipzig: G. Thieme.
v. Monakow, C. 1905. “Gehirnpathologie,” 2 te Auflage. Wien: A. Hólder.
Déjírine, J. 1894. “Anatomie des Centres nerveux,” tome i. Paris: J. Rueff.
Redlich, E. 1905. Arb. a. d. Neurol. Inet. a. d. Wiener Univ., 12: 109.
Reil, T. C. 1809. Arch. f. Physiol., 9: 136.
Burdach, 1819-1826. “Vom Baue und Leben des Gehirns.” Leipzig.
Hultkrantz, J. W. . 1929. “Gehirnpräparation mittels Zerfaserung.” Berlin: J. Springer.
Rosett, J. 1933. “Intercortical Systems of the Human Cerebrum.” New York: Columbia Univ. Press.
Flechsig, P. 1920. “Anatomie des menschlichen Gehirns und Rückenmarks.” Leipzig: G. Thieme. (Here further references.)
Pfeifer, R. A. 1925. “Myelogenetisch-anatomische Untersuchungen über den zentralen Abschnitt der Sehleitung.” Berlin: J. Springer.
Niessl von Mayendorf, E. 1903. Arch. f. Psychiat., 37: 537.
Polyak, S. 1933. “The Main Afferent Fiber Systems of the Cerebral Cortex in Primates.” Berkeley, Cal. : U. of California Press.
Brouwer, B. 1936. “Chiasma, Tractus opticus, Sehstrahlung und Sehrinde.” In: “Bumke-Foerster, Handbuch der Neurologie,” vol. vi. Berlin: J. Springer.
Edinger, L. 1902. Arch. f. klin. Medis., 73: 304.
Bucy, P. C., and H. Klüver. 1940. Arch. Neurol. Psychiat., 44: 1142.
Mingazzini, G. 1922. “Der Balken.” Berlin: J. Springer.
van Valkenburg, C. T. 1913. Brain, 36: 119.
de Villaverde, J. M. 1924. Trav. du lab. de rech. biol. de l'Univ. de Madrid, 22: 99.
Polyak, S. 1927. J. Comp. Neurol., 44: 197.
Biemond, A. 1932. Proc. Acad. Sci. Amsterdam, 35: 1166.
Mettler, F. A. 1935 J. Comp. Neurol., 61: 221.
Sunderland, S. 1940. J. Neurol. Psychiat., London, 3: 9.
Pines, L. J., and B. M. Maiman. 1939. Arch. Neurol. Psychiat., 42: 1076.
Curtis, H. J. 1940 J. Neurophysiol., 3: 407.
Bailey, P., H. W. Garol and W. S. McCulloch. 1941. J. Neurophysiol., 4: 564.
Foerster, O. 1936. “Sensible corticale Felder.” In : “Bumke-Foerster, Handbuch der Neurologie,” vol. vi. Berlin: J. Springer.
v. Bonin, G. 1941. J. Comp. Neurol., 75: 287.
For further research:
Wordcloud: Appear, Area, Association, Boundary, Brain, Brodmann, Bundle, Callosal, Cells, Chimpanzee, Connections, Cortex, Degenerated, Described, Different, Electrodes, Fact, Fibers, Fig, Figure, Firing, Functional, Hemisphere, Histological, Human, Indicated, Investigated, Large, Lobe, Macaque, Man, McCulloch, Method, Monkey, Neurol, Observed, Occipital, Parastriate, Physiological, Point, Preoccipital, Primates, Results, Stratum, Striate, Strychninization, Study, Sulcus, Temporal
Keywords: Lobe, Area, Studies, Cortex, Primate, Structure, Fact, Magno-Cellularis, Chimpanzee
Google Books: http://asclinks.live/ru3h
Google Scholar: http://asclinks.live/r7jn
Jstor: http://asclinks.live/488j