SENSORY CORTEX OF CHIMPANZEE12 [38]
Percival Bailey, J.G. Dusser de Barenne, Hugh W. Garol and W.S. McCulloch
Introduction
Recently two of the present authors (1) gave a preliminary report on the location, extent and subdivision of the sensory cortex of the chimpanzee based on experiments on four animals. We can now present the results of new experiments on five more chimpanzees. The technique used was essentially the same as that of the experiments of the preliminary paper, namely local strychninization of the cerebral cortex in the fully anesthetized animal and investigation of the distribution of the “strychnine spikes” appearing in the electrocorticograms (ECGs) of various portions of the cerebral cortex. The only difference is that in these new experiments thirty-six electrodes were placed on the cortex instead of six as in the previous experiments. Obviously this resulted in much more detailed information. What needs elucidation is how the location, extent and functional organization of the “sensory” cortex can be determined in experiments such as these, in the fully anesthetized animal.
For more than thirty years the use of local strychninization of the central nervous system (CNS) has proved itself an almost ideal method for the study of the location and functional organization of sensory systems in the CNS. In 1909-1913 the senior author (2) applied this method to the spinal cord. In 1915 he (3) found that local strychninization within a certain region of the cerebral cortex of the cat, after recovery of the animal from the operative anesthesia, results in transient—for about 30 minutes—typical symptoms of sensory excitation: paraesthesiae and hypersensitivity of the skin and deeper structures. Strychninization without this region did not result in sensory disturbances. In 1923 the same author (4) using the same methods, namely local strychninization in conjunction with “clinical” observations of the animal, delimited the sensory cortex of the macaque monkey. The obvious conclusion from these two series of experiments is that the region thus delimited subserves somatic sensory functions, is the “sensory” cortex.
After the “sensory” nuclei of the thalamus opticus of the cat had been diagnosed by the same physiological methods (5), Dusser de Barenne and McCulloch (6) showed that local strychninization of the sensory cortex, as ascertained by the first combination of methods, results—even in the fully anesthetized animal—in activation of these very same thalamic nuclei: local strychninization of the various subdivisions of the “sensory” cortex results in the appearance of large and rapid voltage fluctuations—"strychnine spikes"—in the electrograms of the corresponding “sensory” nuclei of the thalamus.
Finally Dusser de Barenne and McCulloch (7) and these authors with Ogawa (8) studied the distribution of the strychnine spikes in the electrograms of the cerebral cortex following its local strychninization in the fully anesthetized monkey. Again the same region was as delimited with the original combination of methods. Local strychninization within this region resulted in widespread strychnine spikes with a typical distribution following strychninization of each constituent area; strychninization without this region never resulted in the appearance of strychnine spikes in the electrograms taken within the region. Furthermore, the distribution of the strychnine spikes is specific for each constituent area of this region. The obvious conclusion from these various pieces of experimental evidence is that the distribution of the strychnine spikes in the cerebral cortex following its local strychninization permits one to determine the location, extent and functional organization of the “sensory” cortex even in the fully anesthetized animal.
“Clinical” observation of as strong an animal as the anthropoid ape after local strychninization of its cerebral cortex would be too dangerous for the experimenter. We have, therefore, for the study of the chimpanzee’s sensory cortex, employed the combination of methods which can be used in the fully anesthetized animal. Again this combination of methods has revealed on the surface of the hemisphere a very large region, the location, extent and functional organization of which is comparable to that of the sensory cortex of the macaque monkey. The obvious inference again is that the region so disclosed is the sensory cortex of the chimpanzee.
On all hemispheres of this new series, except one, extensive electrical stimulations of the cortex were performed with special stimulators (see the section on methods) allowing the use of various pulseforms. The relevant findings of these stimulation experiments and their correlation with the findings in this paper will be published in a separate paper.
Methods
These experiments were performed on 5 immature, 2.5 to 3.5 years old, chimpanzees (Pan satyrus), fully anesthetized with Dial3 (0.35 —0.45 cc. per kg. body weight, half of the doses given intraperitoneally, half intramuscularly). When the animal was fully under narcosis the head was clamped in a special head holder, and the body placed on an inclined board so that the head was lower than the hindquarters of the animal; this was done to maintain proper cerebral circulation.
Each investigation on one animal lasted from 3 to 3.5 days without interruption. It was, therefore, necessary to work in day- and nightshifts, usually from 12 :00 noon until 12:00 midnight and from 12 :00 a.m. until 12:00 p.m. 4 During the first 1.5 to 2 days the sensory cortex of one hemisphere was investigated, the rest of the time was devoted to the exploration of the second hemisphere.
After exposure of the larger part of the convexity of one hemisphere several photographs of the exposed region were taken from different angles to get as little perspective distortion as possible in the final composite drawing of the hemisphere. Lifesize prints were made to record accurately the location and extent of the several local strychniniza-tions and of the electrically excitable “motor” cortex.
The investigation of each hemisphere was begun with a careful exploration of the “motor” cortex. For this a specially constructed stimulator designed and built by Mr. Craig W. Goodwin, the electronic engineer of this laboratory, was used.5 This apparatus allows independent variation of the rising and falling phases of each individual pulse and the frequency of the pulses per second, the pattern-frequency of the stimulation, within a wide range, and the number of pulses per stimulation. For several years Dusser de Barenne and McCulloch had known (from unpublished experiments) that with long pulses—of several sigmas’ duration—the voltage required to stimulate the “motor” cortex is much lower than with short pulses and that especially the duration of the descending phase seems significant; under moderate Dial-anesthesia a descending phase of from 8 to l2σ duration is optimum. Where necessary, due caution was taken in regard to the disturbing influence of facilitation and extinction (9). In other instances facilitation was used as a useful factor in determining the most frontal boundary of the “motor” cortex. An important feature of the stimulatory exploration was also the delimitation of the boundaries between the leg-, arm- and face-subdivisions of the “motor” cortex. For further details see the next paper on the “motor” cortex of the chimpanzee.
After the careful exploration and mapping of the “motor” cortex the experiments with local strychninization were begun. Because of the tremendous functional complexity of the chimpanzee’s cortex it was decided to concentrate our efforts in this group of animals mostly on the arm-subdivision; in the last chimpanzee attention was focused predominantly on the leg-subdivision. Six columns of 6 stigmatic Ag-AgCl2 electrodes were placed on the exposed portion of the cortex, the uppermost of each column high up in the leg-subdivision, dorsal to the boundary between the leg- and arm-subdivisions; the 5 others of each column were spread vertically over the dorso-ventral extent of the arm-subdivision. In several of the experiments the most ventral electrode of each column was placed in the face-subdivision. Thus, although in these experiments most information was obtained about the arm region of the sensory cortex, a good deal of evidence about the leg-and face-regions and the functional boundaries between the three major subdivisions of the sensory cortex was also acquired. On the precentral cortex the results of the electrical explorations gave useful information for the placement of the electrodes; on nearly all of the postcentral cortex this guiding factor was not available and we relied on experience with the functional boundaries obtained in the chimpanzees of the preliminary paper.
The wires from all 36 electrodes were connected to a 6 pole-6 throw switch and from this to the 5 amplifier sets of 5 Grass inkwriter-oscillographs in such a way that in one position of the switch all 6 electrodes of one column were connected to the oscillographs. Thus, by turning the switch, each column of electrodes on the cortex could quickly be connected with the amplifiers and oscillographs. If we call the 6 electrodes in each column from above downwards, a, b, c, d, e and f and the 5 oscillograph channels A, B, C, D and E, the hook-up was such that electrodes a and b were connected to channel A, electrodes b and c to channel B. . ., electrodes e and f to channel E. A change or disturbance of the electrical activity in the cortex under or near electrode a will affect only channel A, stimultaneous and inverted strychnine spikes or any other change in the ECGs in channels A and B signifies a disturbance under or near electrode b, simultaneous changes in channels C and D a disturbance under or near electrode d . . . and a change in the ECG of channel E by itself a disturbance under electrode f. Thus each change in the ECGs of any channel or group of channels reflects a change in the activity of the cortex under or near a particular electrode or group of electrodes. Thus, in turning the 6 pole-6 throw switch one can investigate in quick selection and succession the ECGs of the cortex under or near the 6 electrodes of each of the 6 columns.
The taking of the ECGs before and after each strychninization constitutes one experiment, whose actual course usually was as follows: (i) the taking as “control runs” for 1 min. of the “normal” ECGs for each of the 6 columns of electrodes, (ii) the local strychninization of the cortex by applying a small piece of filterpaper of appropriate size and shape soaked in a 3 per cent solution of strychnine sulfate colored with toluidine blue; (iii) the taking of half-minute runs of ECGs for each of the 6 columns of electrodes in succession until any change, if such resulted from the strychninization, had passed off. Five to ten minutes after its application the filterpaper was removed and any fluid at the site of strychninization carefully blotted. Usually after 25 to 45 minutes the effects of such a strychninization had passed off, depending upon the depth of narcosis, the circulation, etc.
In our work on the macaque’s cortex truly local strychninizations were always performed, i.e., strychnine-filter papers of a few square millimeters applied. In the chimpanzee the effects of such local strychninizations are very much more restricted than in the
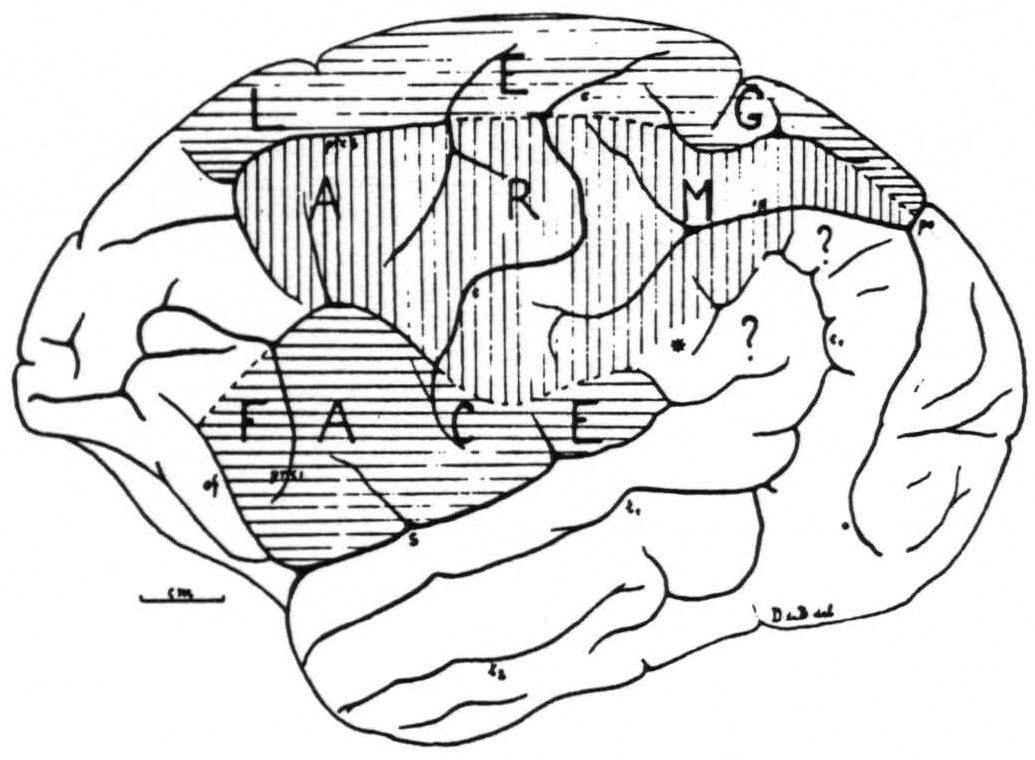
Figure 1. Sensory cortex of the chimpanzee. This figure represents as accurately as is possible in one plane the outer surface of one hemisphere of chimpanzee No. 4. The precentral boundary between the face- and arm-subdivisions lies in this hemisphere unusally low, but was verified both by electrical stimulation with motor response and with the strychnine method. It is also unusual that this boundary is marked by a definite sulcus across the precentral gyrus. The figure schematizes the results of over 200 observations on this one brain.
macaque; therefore, the strychnine filterpapers used in the chimpanzee experiments were taken larger, 3×5 or 2×10 millimeters.
Thus, in general, during each experiment from 8 to 14 series of ECGs from the 6 columns of electrodes were taken. Usually between 15 to 25 strychninizations were performed on one hemisphere, i.e., 30 to 50 experiments performed on the brain of each chimpanzee.
At the end of such an investigation, lasting without interruption for 3 to 4 days, the brain was injected through the carotids with 15 per cent neutral formalin, then removed from the skull and placed in formalin. After 12 to 24 hours the brain was weighed and carefully measured. After a few days the soft membranes were peeled off and the brain photographed before and after removal of the brainstem and cerebellum and after splitting in the midplane. Finally all the strychninizations were carefully mapped on life size photographs of the hemispheres.
Then the analysis of the several thousand feet of record was begun and the change of the ECG under each electrode in each experiment marked on drawings (twice life size) of each hemisphere (“firing,” suppression, “spindles,” increase of activity, no change). Thus the final plotting of the results of each experiment on the cortex of one hemisphere is based on the study of circa 400 ECGs (each comprising 8 or more records) and the final composite diagram, on that of well over 100,000 records.
Results
Location, extent and subdivision of sensory cortex
The experiments of this new series have essentially confirmed the location and extent of the sensory cortex of the chimpanzee as given in the preliminary report and as reproduced in Fig. 1. They have permitted us to answer a few of the questions left in this diagram. Those small areas marked there with a ? do not belong, as we can definitely state now, to the sensory cortex.
We are also in a position now to state that the small triangular area
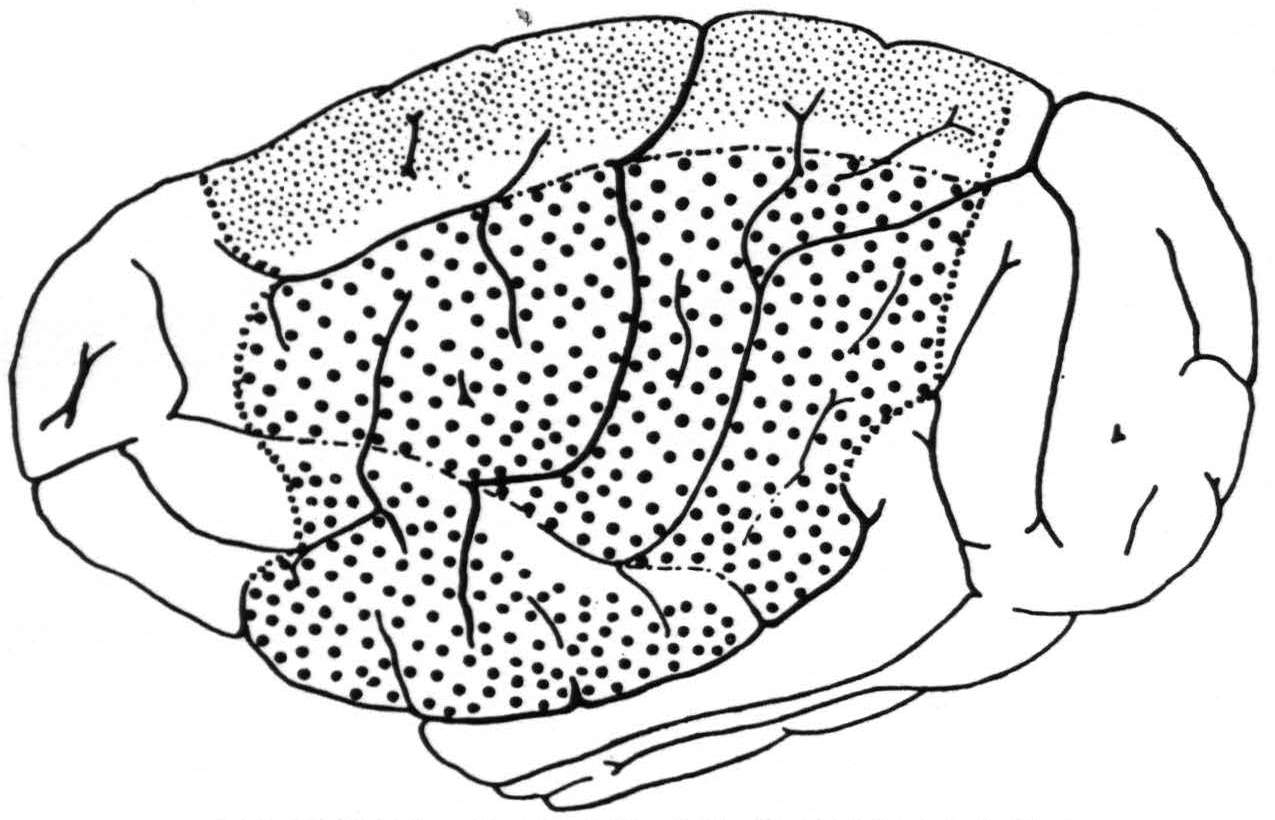
Figure 2. A composite drawing showing the extent and location of the sensory cortex and its subdivisions for leg ( ), arm (
), arm ( ) and face (
) and face ( ). The regions for trunk (between arm and leg) and for neck (between arm and face) are left blank.
). The regions for trunk (between arm and leg) and for neck (between arm and face) are left blank.  = anterior and posterior margins of the sensory cortex; —•—• =boundaries of arm-subdivision.
= anterior and posterior margins of the sensory cortex; —•—• =boundaries of arm-subdivision.
marked with an * in the first diagram belongs to the arm-subdivision. The posterior border of the sensory leg cortex did not extend in the last 5 animals all the way to the fissura parieto-occipitalis externa (ope), as was the case in the fourth animal of the first series; apparently individual variations are not infrequent.
In Fig. 2 is given an “average” (see discussion) aspect of the convexity of the chimpanzee’s brain; in it are indicated also the location, extent and subdivision of the sensory cortex as delimited in these new experiments. It will be seen that this cortex occupies a large portion of the hemisphere on its outer surface both before and behind the fissura centralis and comprises three major subdivisions: the leg-, arm- and face-subdivisions. Between the leg- and arm-subdivisions lies a narrow strip of cortex in which presumably the trunk is represented sensorially. This statement is based on (i.) the existence in the precentral cortex of an intermediate “motor” trunk region and (ii.) the occurrence of small or questionable strychnine spikes within both the pre- and postcentral portion of this intermediate region from strychninizations definitely in the leg- or arm-subdivision. Between the arm- and face-subdivisions lies a narrow strip of cortex in which presumably the neck is
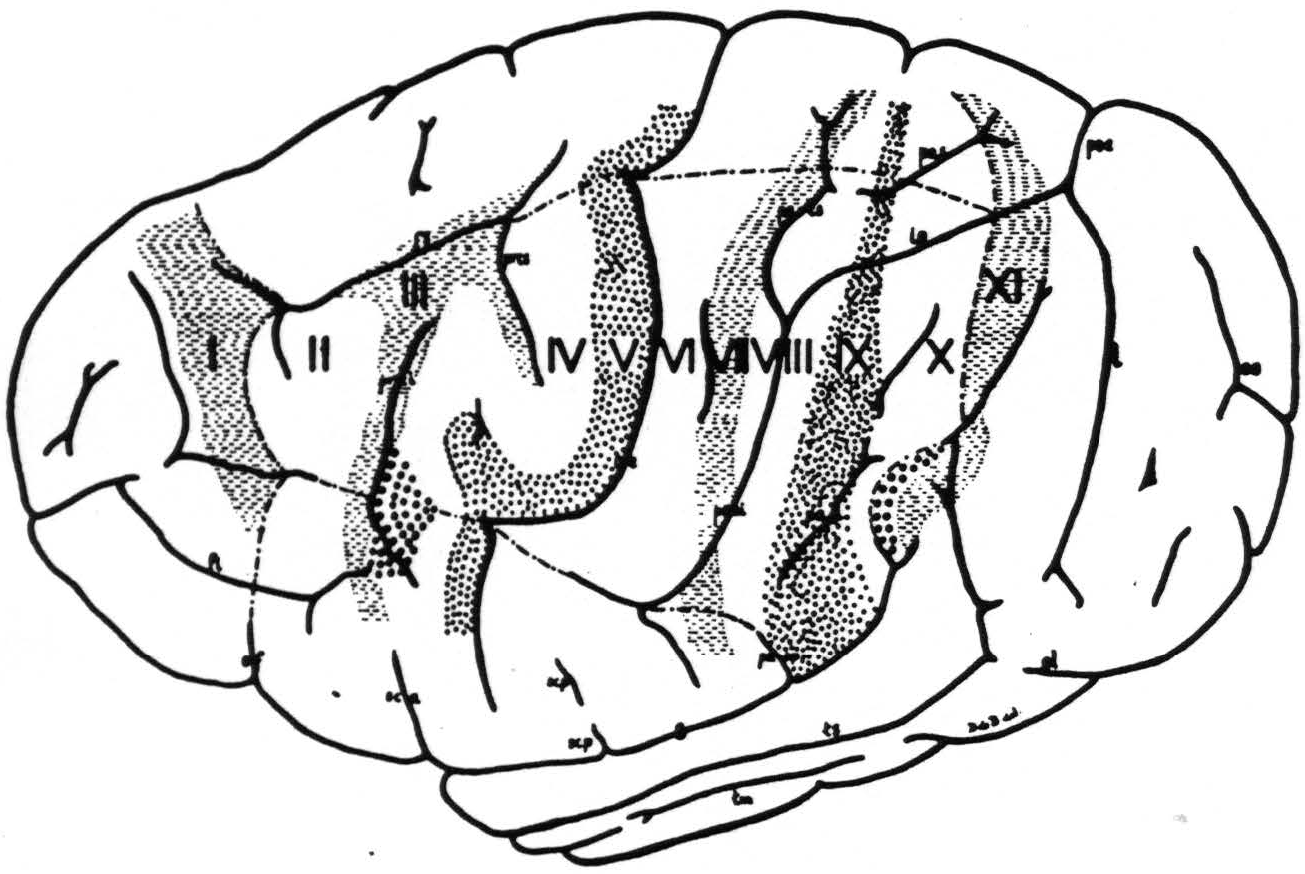
Figure 3. The extent, location and functional subdivisions of the sensory arm cortex into physiologically distinguishable bands, Nos. II-X, and of the immediately adjacent bands, Nos. I and XI, indicated on a composite drawing representing the arm area in the center of the field. The bands giving suppression, Nos. I, III, VII and XI, are marked thus: ≡ ≡ ≡. Small  indicate bands V and IX. Large
indicate bands V and IX. Large  mark the “dud” areas. Areas between trunk and arm and between arm and neck are marked —•—•.
mark the “dud” areas. Areas between trunk and arm and between arm and neck are marked —•—•.
represented sensorially. The reasons for this statement are the same as for the trunk region, mutatis mutandis.
While we refer to this region as the “neck” region it should be remembered that in these experiments the head of the animal was fixed, so that the only musculature about whose contraction definite observations could be made was the superficial neck musculature: platysma, sterno-cleido, splenius capitis, pinna muscles, etc. The boundaries of these two intermediate regions are drawn in Fig. 2 as more distinct towards the arm-subdivision because we have enough information to establish these margins fairly accurately.
Functional organization of sensory cortex
In the previous work on the macaque’s sensory cortex it was found that the distribution of the strychnine spikes following local strychninization of some area is not only beyond the limits of this area but follows a definite “pattern” for each area. This means, as has been established by further investigations (10), that the various areas of the sensory cortex of the macaque monkey have different interareal neuronal connections. Thus this combination of methods reveals what we have called the “functional organization” of the macaque’s sensory cortex
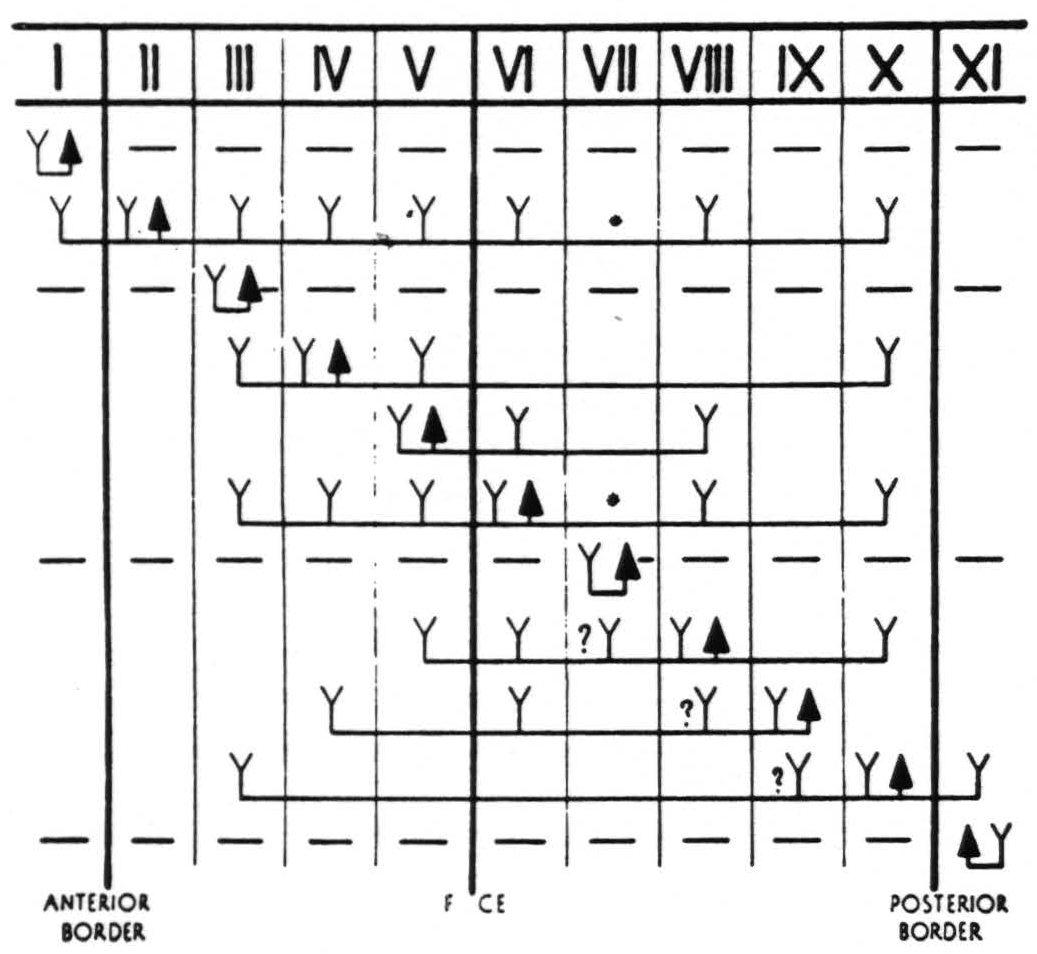
Figure 4. In Figure 4 are represented diagrammatically the directed functional (and anatomical) relations between the various cortical bands of the arm-subdivision of the sensory cortex found in these experiments and also those of bands I and XI adjacent to but outside of the sensory cortex. Anterior and posterior borders are the limits of the sensory cortex. The suppression of the ECG of various bands upon strychninization of bands I, III, VII and XI is indicated thus: ———.
F CE =fissure centralis; * =no certain evidence; ?Y =definite “firing” but uncertainty whether strychnine invaded region so “fired.”
The same is true for this cortex of the chimpanzee, but with differences: (i) with truly local strychninization the distribution of the strychnine spikes is in this animal much less widespread than in the macaque, sometimes confined to a relatively small region in the neighborhood of the site of strychninization; (ii) when the spikes are present in another more or less remote region, only a small portion of that region is involved.
Concentrating attention on the arm-subdivision it was found to be composed of several functionally dissimilar vertical “bands” of cortex, characterized by: (i) the unique distribution of the strychnine spikes following strych-ninization within any one band; (ii) the appearance of strychnine spikes within it following strychninization of other bands.
Unfortunately the only complete cytoarchitectonic map of the chimpanzee’s cortex is that of Campbell (11), which fails to show many differentiations found functionally in these experiments. Furthermore, the other available cytoarchitectonic studies on the precentral areas 4 and 6 of the chimpanzee (12, 13) differ so widely from Campbell’s findings, as to be of little help here. We have, therefore, abstained from any attempt to correlate anatomy and physiology here and constructed our diagram so as to indicate only the physiological differentiation met in our experiments. We have, therefore, assigned to each functionally unique band so revealed an arbitrary number, I through XI. Bands II through X comprise the sensory arm region, whereas bands I and XI (see below) lie without it. Figure 3 presents the location and extent of these bands, mainly in the arm-subdivision, on the chimpanzee’s brain.
While these bands indicate greater differentiation in the chimpanzee’s sensory cortex than in the macaque’s, there appear certain similarities to the functional organization of this animal’s sensory cortex as indicated below. Figure 4 presents the functional organization of the arm-subdivision of the chimpanzee’s sensory cortex.
The most anterior band of the chimpanzee’s sensory cortex, No. II, like area 6 of the macaque, shows the widest distribution of the strychnine spikes, “firing” practically all of the sensory arm cortex except band IX and being fired by no other band than itself. It should be noted that, like area 6 of the macaque, local strychninization of arm band II “fires” not only the arm-subdivision but also the leg-subdivision and even the face-subdivision. It should further be noted that strychninization of band II also “fires” band I, outside the sensory cortex.
Band III has properties comparable to those of area 4-s in the macaque, in that it gives pure “suppression” of electrical activity of other regions of the sensory cortex and spikes only itself.
Bands IV and V together “behave” functionally like area 4 of the macaque. Local strychninization of band VI results in widespread strychnine spikes in both the pre- and postcentral arm cortex; it does not “fire” band II, or band IX.
Band VII is again a suppressor region comparable to the postcentral suppressor area in the macaque’s brain and spikes only itself.
Bands VIII, IX and X together resemble the sensory cortex of the macaque’s cortex behind its postcentral suppressor area. Whereas local strychninization of bands VIII and IX respect functional boundaries, this is not the case with band X. Local strychninization of leg X “fires” not only itself but also arm X and leg and arm III, local strychninization of arm X “fires” not not only itself but also leg X and leg and arm III.
These are the essential functional similarities of the sensory cortices of chimpanzee and macaque; now as to the dissimilarities.
The outstanding difference is that whereas in the macaque when spiking occurs in an area of the sensory cortex it generally involves all parts of this area, even though the strychninization is truly local, the spiking upon strychninization within a band of the chimpanzee’s cortex is much more restricted even when the area strychninized is much larger. Even with 36 electrodes on the cortex and relatively large strychninizations one cannot hope to obtain the complete distribution of strychnine spikes from a given area in any single experiment. This is not and cannot be represented in Fig. 3 and 4, which are composites of all strychninizations in all 6 animals and show the greatest distribution obtained. What does appear in these figures is the greater differentiation into more bands than areas that could be distinguished in the macaque’s sensory arm region, witness the differentiation of the immediate precentral cortex in the chimpanzee, subdivided into bands V and IV, (together comparable to area 4 of the macaque) and the far post-central bands VIII, IX and X. Characteristic of the chimpanzee’s sensory cortex is the finding of “firing” of remote regions without “firing” of some intermediate region or regions: the failure of bands II and VI to “fire” band IX, though both “fire” band X; the failure of band IV to “fire” bands VI, VII, VIII, and IX, though it “fires” band X; the failure of band V to “fire” VII, though it “fires” VIII; the failure of band VIII to “fire” band IX, though it “fires” band X; the failure of band IX to “fire” bands V and VII, though it “fires” bands VI and IV, and finally the failure of band X to “fire” bands VIII, VII, VI, V and IV, though it “fires” band III.
An interesting finding is that in the precentral sensory cortex (see Fig. 3) lies a “dud” area, “dud” in this sense, that its strychninization “fires” no other area and that it is “fired” by strychninization of no other area. Above the end of the fissura Sylvii, bulging into the sensory cortex, but not part of it, lies a second “dud” area in the same sense as the first area (see Fig. 3).
Anterior and posterior to the sensory arm cortex were found two bands, I and XI respectively, the local strychninization of which results in a marked suppression of the electrical activity of a great part of the convexity of the hemisphere. Local strychninization of band I results not only in suppression of the electrical activity of the whole arm cortex, but also of that of the leg-, trunk-, neck- and face-subdivisions and of that of band XI; local strychninization of band XI similarly gives suppression of the electrical activity of the entire sensory cortex and of that of band I. It is interesting to note that this widespread suppression from these two areas does not occur simultaneously in all bands of the sensory cortex, but slowly, in the course of many minutes, sweeps across this cortex in a definite sequence, the bands nearest to that strychninized “going under” first, those more remote later. (See Fig. 5 and 6)
Figure 6 gives the right hemisphere of chimpanzee VI on which this particular experiment was performed to obtain the records of Fig. 5 and the position of the electrodes with the changes of the electrical activity of the cortex underneath each of these electrodes ( + for “firing,” — for suppression, 0 for no change), and the site and extent of the strychninization.
Very interesting is the finding that the sequence of the bands is more orderly
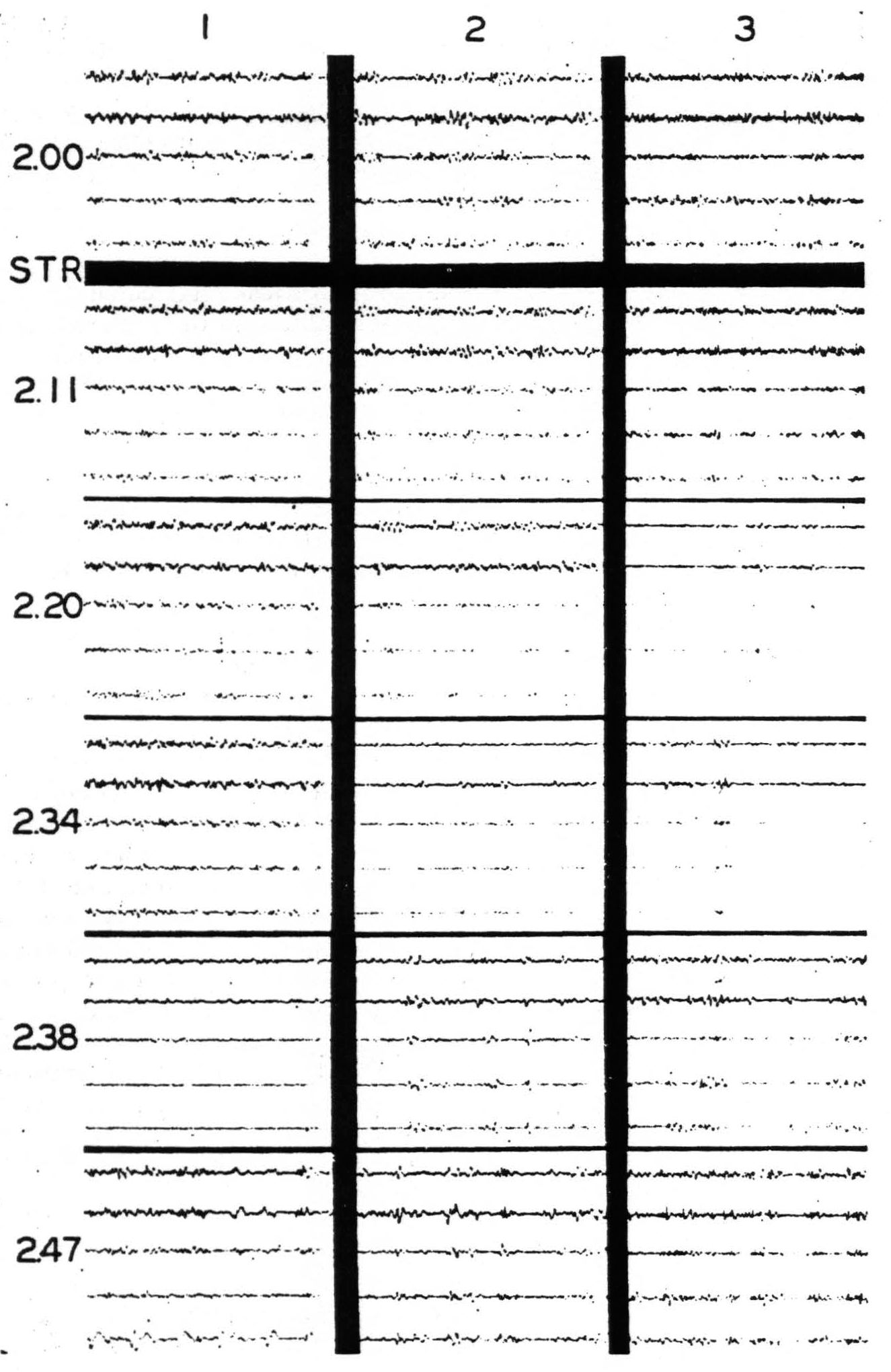
Figure 5. Nov. 7, 1939. Chimpanzee VI. Right hemisphere. Second day. Strychninization No. 18 at S in band XI (see Fig. 6) at 2:04 p.m. between the recording of the ECGs of rows 1 and 2. (For arrangement of electrodes on cortex and on records and their connections with amplifier oscillograph sets see Fig. 6.) Note the “firing” in the ECGs coresponding to electrodes b, c,d and e of column 6, and the “sweep” of the suppression of the electrical activity across the cortex.
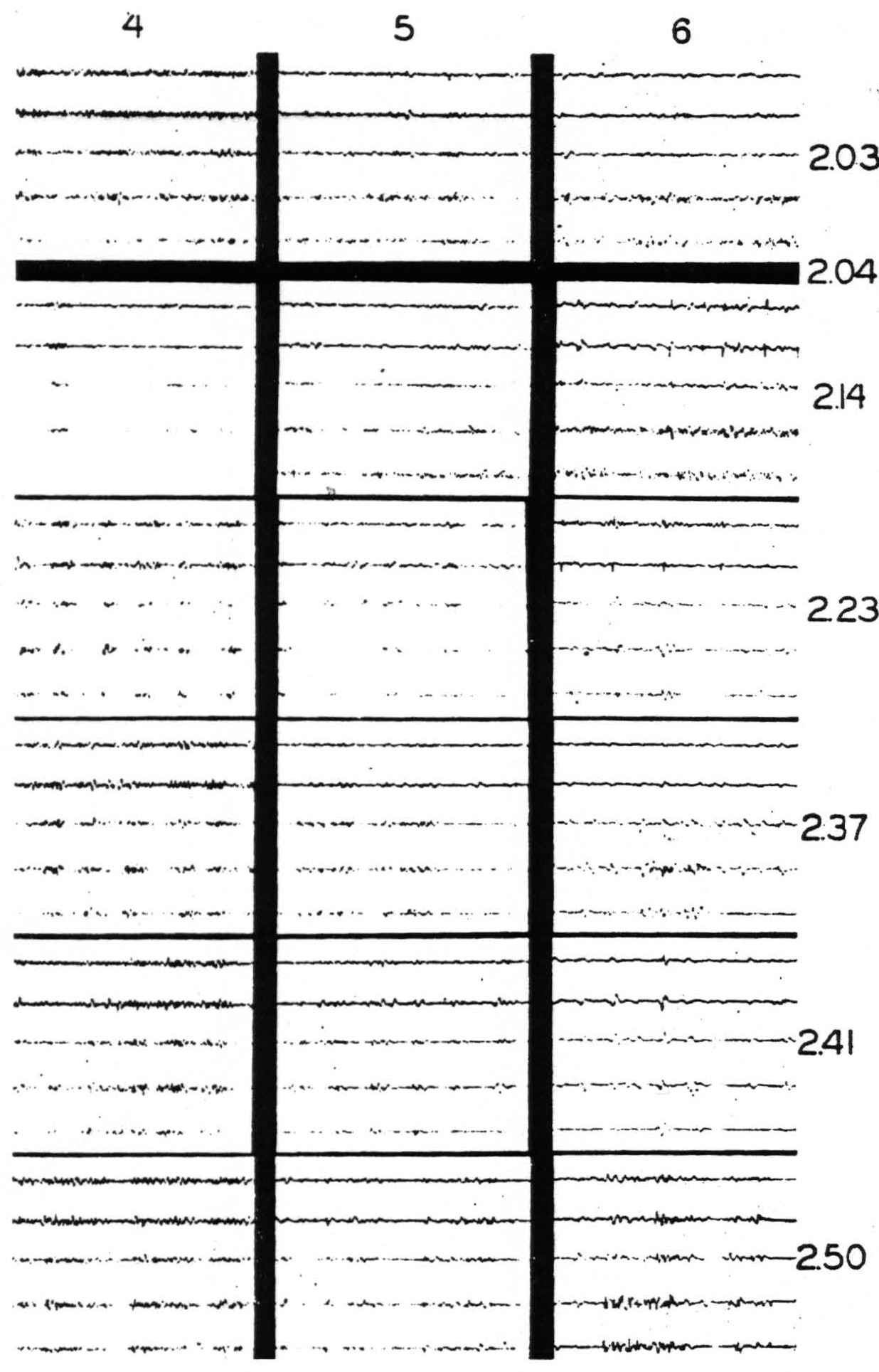
Figure 5. Nov. 7, 1939. Chimpanzee VI. Right hemisphere. Second day. Strychninization No. 18 in band XI (at S in Fig. 6 and indicated by STR in Fig. 5) at 2:04 p.m. between the recording of the ECGs of rows 1 and 2. (For arrangement of electrodes on cortex and on records and their connections with amplifier-oscillograph sets see Fig. 6.) Note the “firing” in the ECGs corresponding to electrodes b, c, d and e of column 6, and the “sweep” of the suppression of the electrical activity across the cortex.
than that of the sulci, i.e., on crossing a sulcus a band may appear interrupted and one of its portions appear displaced anteriorly or posteriorly depending upon the angle between the band and the sulcus on the surface. For instance, if in a particular brain the “spur” of the first temporal sulcus bends anteriorly, the portion of band XI above this spur lies anterior to its continuation below the spur, whereas in an animal in which the spur of the first temporal sulcus bends posteriorly, the superior portion of band XI lies
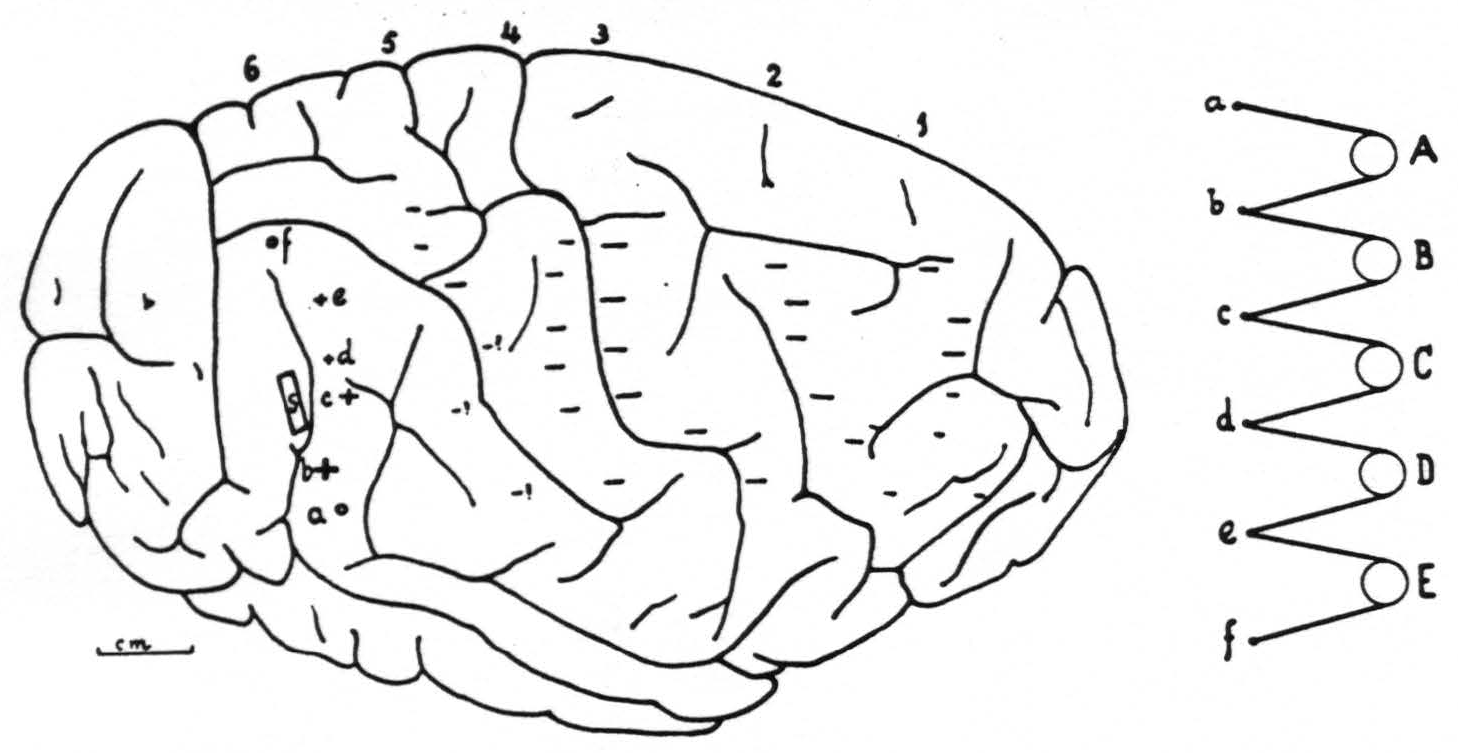
Figure 6. Right hemisphere of chimpanzee VI with location and extent of strychninization (S), positions and arrangement of the 6 columns of 6 electrodes and the change in electrical activity under each electrode ( +for "firing,"— for suppression, 0 for no change). This figure refers to the experiment recorded in Fig. 5. Notice that the arrangement of electrodes on cortex and in records is inverted. a, b, c, d, e and f represent the 6 electrodes of any column, A, B, C, D and E the 5 amplifier-oscillograph sets.
posterior to its inferior, or ventral, portion. This finding must mean that the location of the bands is more fundamental than the location and configuration of the sulci crossed by the bands.
Discussion
The most salient point which these new investigations have brought out, just because of the great amount of information obtained in each hemisphere, is that it is almost impossible to compose a reliable diagram of the location, extent, subdivision and functional organization of “the” chimpanzee’s sensory cortex. The variability in the configuration of this animal’s cortex is so great as to preclude any definite homologization of any but the principal fissures and sulci. The size, shape, position and direction of the secondary sulci is so variable that these (sometimes, even) often, cannot be identified with certainty. Nevertheless it was necessary to use some composite diagram of the external configuration of the hemisphere. After a careful study not only of the hemispheres in our own collection but also of the available material in the literature (Retzius, Mingazzini and others) the composite diagram presented in Fig. 2 and 3 was made. This is believed to represent not too unfaithfully the “average” external aspect of the chimpanzee’s brain. Since in nearly all of these studies attention was focused on the arm-subdivision, this diagram was composed from photographs centered upon the middle of this subdivision. Given the great variability of the external configuration of the chimpanzee’s hemisphere it will be readily understood that the sites of individual strychninizations and electrodes are extremely difficult to homologize from hemisphere to hemisphere. Although these sites were in each instance plotted on photographs and drawings of that hemisphere on which the particular experiment was performed, it was found most practicable for the final synopsis to transfer them to sites homologized as well as possible on the composite diagram.
The difficulties mentioned are especially great with respect to band VII because it is narrow and occupies the posterior margin of the extremely variable postcentral gyrus. When this gyrus is wide and has a longitudinal sulcus on its convexity band VII is found as indicated in Fig. 3; when the gyrus is narrow it is sometimes impossible to find band VII in the central position of the arm-subdivision and, if found, it may appear immediately behind the postcentral sulcus. Most difficult are those hemispheres in which the postcentral gyrus is narrow and the junction of the superior postcentral sulcus with the intraparietal sulcus is low down, far forward and complicated in form while the longitudinal dimple on the postcentral gyrus is absent or at least does not appear as a separate sulcus. This variability is so great as to make it almost impossible to place electrodes willfully in this particular band. The diagram of functional organization (Fig. 4) shows, therefore, the most probable changes in activity following the various strychninizations with electrodes probably placed in band VII. The asterisks indicate complete uncertainty in this respect. In chimpanzee 9 we have evidence that if electrodes are placed in the leg region of band VII, strychninization of band II and of band VI “fires” band VII. We are, therefore, inclined to believe that the same is true of the arm-subdivision, where evidence is still to seek.
Similar difficulties arise especially for the more dorsal part of band IX, where it narrows down as it passes into the trunk region, and the variability of the ends of the inferior parietal sulcus, the fissura Sylvii and the first temporal sulcus complicates the picture. However, in the case of band IX enough experimental evidence (sites of electrodes and strychninizations) was available to overcome, in our opinion, the difficulties.
The question marks in Fig. 4 do not indicate uncertainty as to “firing” or position of the electrodes in these bands, but signify that the strychnine may have extended into the region so fired from that adjacent region to which it was applied. In each case the firing marked with a ? was only in the proximity of the strychninization and did not involve more remote parts of the band under consideration.
Not indicated in Fig. 4 are: that in chimpanzee 9 strychninization of band XI in the leg region, besides “firing” itself, suppressed itself, and that in one instance strychninization in band I, besides “firing” this band, questionably suppressed the same. We shall now discuss the bands seriatim.
Band I. This band lies outside the sensory cortex by the criterion that strychninization within it never “fires” any portion of the sensory cortex. Its primary characteristic is that its strychninization gives suppression of cortical electrical activity, which may involve the entire sensory cortex and even extend beyond it. This suppression does not respect functional boundaries between the major subdivisions nor is it present simultaneously in all parts of the cortex, but sweeps across it from before backward. Thus strychninization of band I may result in suppression of the leg-, arm- and even face-subdivisions, starting in the intermediate precentral cortex, then appearing in the immediate precentral, then in the immediate postcentral and finally in the parietal cortex. The second characteristic of band I is that it is “fired” by strychninization of no part of the sensory cortex except band II. Band I apparently is comparable to that area, lying in front of L. and A. 6 of the macaque’s brain, from which eye movements can be elicited and the local strychninization of which gives suppression of the electrical activity within the sensory cortex (15).6
Band II. The primary characteristic of this band is that it is a region, whose strychninization results in the most extensive “firing” obtainable from any region of the sensory cortex. Not only does it “fire” the entire pre-central cortex and the entire postcentral sensory cortex, except for band IX, but it also fires band I and fails to respect functional boundaries. For instance: Face II can “fire” even leg X; arm II can “fire” the whole of the arm-subdivision (except IX) and portions of the leg- and face-subdivisions.
It is perhaps an oversimplification to treat band II as a single entity, for in a given hemisphere one can obtain from some portions of band II the “firing” of band X without that of the intermediate regions and by strychninization of another pertion of band II “firing” of the intermediate region without “firing” of band X and by strychninization of other portions an admixture of these two results. However, the present evidence does not allow us to make any more definite statements on this score.
Band II is the most anterior portion of the sensory cortex by the criterion used. It is obviously comparable to area 6 of the macaque’s sensory cortex, with respect to the distribution of the strychnine spikes, especially the failure to respect functional boundaries.
Band III. This band is a suppressor region, i.e. its strychninization gives a widespread suppression of electrical activity in the sensory cortex and in bands I and XI. The effect on itself is an initial spiking followed by suppression of its electrical activity. The suppression obtainable from this band violates functional boundaries. Its properties and its position immediately behind band II indicate its homology to area 4-s of the macaque’s sensory cortex. An apparent difference from area 4-s of the macaque is that, in that animal, we were never able to obtain suppression without “firing” of postcentral areas, whereas in the chimpanzee we have obtained pure suppression in 2 out of 15 cases of suppression from band III. Moreover, the “firing” of some of the regions in the cases of mixed suppression from band III was clearly referable to extension of the strychnine into the adjacent bands.
Bands IV and V. These two bands occupy the cortex between band III and the fissura centralis. In their primary characteristics these bands have in common that their strychninization results in “firing” of other areas, not in suppression. It is, however, necessary to distinguish two bands in this region, band IV and band V, because the strychninization within the frontal portion, band IV, gives a distribution of strychnine spikes different from that following strychninization within the posterior portion, band V, and because the distribution of the strychnine spikes following strychninization of some of the postcentral bands (VIII and IX) is also different in regard to bands IV and V. (See Fig. 4.) The position of bands IV and V is roughly comparable to area 4 of the macaque and a simultaneous local strychninization of the two of them gives results closely comparable to those following local strychninization of the macaque’s area 4. Moreover, these bands IV and V are that region of the cortex of the chimpanzee, ordinary faradic stimulation of which elicits prompt, discrete movements, and which cytoarchitectonics pronounces to be the area giganto-pyramidalis. Thus, in the chimpanzee it has been necessary to make, in this region, a distinction which thus far we have not been able to discern in the macaque.
Band VI. This band is a “firing” band. Strychninization within this band besides “firing” itself can “fire” bands III, IV, V, VII, VIII and X. We have never observed any “firing” of bands II or IX following strychninization of VI. The “firing” obtainable from this band is the most extensive of the “firings” obtainable from the postcentral censory cortex.
Band VII. Strychninization of this band results in suppression of electrical activity throughout the entire sensory cortex and even outside—bands I and XI. The electrical activity of band VII itself shows an initial “firing” followed by suppression. In 2 out of 13 cases pure suppression was obtained.
Band VIII. This band is a “firing” band, affecting of the precentral cortex only band V.
Band IX. This again is a “firing” band, which can be differentiated from band VIII in that its strychninization does not “fire” band V, but “fires” band IV. With respect to the postcentral cortex, it should be noted that band IX does not “fire” X, whereas strychninization of band VIII does “fire” X. Moreover, band IX is unique in the postcentral sensory cortex insofar as it is “fired” only by strychninization within it. The question mark re “firing” of this band from band X denotes uncertainty whether the strychnine responsible for this “firing” of the latter band had not transgressed into band IX.
Band X. Again this is a “firing” band, strychninization of which “fires” only band III of the precentral cortex and, of the postcentral sensory cortex, only itself. This band of the postcentral sensory cortex is unique in that its “firing” does not respect functional boundaries, i.e., does not remain within the subdivision locally strychninized. Strychninization of leg X can “fire” leg X and arm X and leg III and arm III. It also “fires” band XI, outside the sensory cortex. We say “outside the sensory cortex” because band X being the most posterior region to “fire” into the sensory cortex, by this criterion, is the most posterior of its constituent bands. With respect to homologizing the postcentral bands with the postcentral areas of the macaque’s sensory cortex we wish to take up the discussion of these bands together, since we have not as yet been able to make a comparable differentiation in the macaque. All we can say definitely at present is that in both the the chimpanzee and the macaque there exists a postcentral sensory suppressor band—band VII of Fig. 3 and 4—behind which lies a region which in both animals “fires” into the precentral sensory cortex. In view of the findings in the chimpanzee the functional organization of the sensory cortex of the macaque’s brain is under reinvestigation with cytoarchitectonic controls. Not until this work is completed, do we dare to say anything in regard to homologization of the more anterior parts of the postcentral sensory region of the chimpanzee and macaque.
Band XI. This band lies outside the sensory cortex by the criterion that strychninization within it never “fires” any portion of the sensory cortex. Its primary characteristic is that strychninization within it suppresses the entire sensory cortex and even band I in front of it.
For the arm-subdivision we have no evidence that strychninization of band XI besides “firing” itself may also later suppress itself; for the leg-subdivision (chimpanzee 9) the sequence of these two phenomena was observed.
In the re-investigation of the macaque’s cortex mentioned above special attention was paid to the question whether in this animal a region outside the sensory cortex, comparable to band XI of the chimpanzee’s brain, exists. The investigation has proceeded far enough to show that this is the case and to state that these bands in the two species correspond closely as to position and shape. This statement holds also for the manguebey’s brain.
It has been found that the posterior border of the sensory cortex in the chimpanzee and, with it, the location of band XI on this brain in relation to the fissura parieto-occipitalis externa and the end of the first temporal sulcus shows great variability. In the chimpanzee on which the diagram of the sensory cortex in the preliminary note (Fig. 1) was based this cortex extended clear to the parieto-occipital fissure. In all the animals upon which this paper is based it was found that the posterior border of the sensory cortex lay in front of this fissure and even that in some hemispheres band XI—outside the sensory cortex—did not extend as far back as this fissure. The same variation has subsequently been found in the macaque. These findings suggest that band XI and its homologue in the macaque may well be area 19 of Brodmann.
Interesting is the posterior “dud” area bulging into the sensory cortex between the end of the fissure of Sylvius and the first temporal sulcus. The diagrams of Campbell (14) for man and the subhuman apes show a region of essentially similar configuration, belonging, according to Campbell, to the temporal lobe bulging into the parietal cortex, the posterior end of his “auditopsychic” area. We wish to point out that it was not until we were writing this discussion and trying to make the homologizations that we became aware of the remarkable coincidence between this point in our physiological findings and Campbell’s histological studies. A similar “dud” area has subsequently been found in the macaque in the vicinity of the end of the fissura Sylvii and anterior to the first temporal sulcus.
In conclusion it should be emphasized that the diagram of the functional organization of the chimpanzee’s sensory cortex as given in Figs. 3 and 4 shows the maximum of “firing” and suppression observed, not in one animal nor following one strychninization, but that it gives the synopsis of all data collected.
Footnotes
REFERENCES
Dusser de Barenne, J. G., and McCulloch, W. S. The sensory cortex of the chimpanzee. Proc. Soc. exp. Biol., N. Y., 1939, 42: 27-29.
Dusser de Barenne, J. G. Die Strychninwirkung auf das Zentralnervensystem II. Zur Wirkung des Strychnines bei lokaler Applikation auf das Rückenmark. Folia neuro-biol., Lpz., 1911, 5: 42-58.
Dusser de Barenne, J. G. Experimental researches on sensory localisations in the cerebral cortex. Quart. J. exp. Physiol., 1916, 9: 355-390.
Dusser de Barenne, J. G. Experimental researches on sensory localization in the cerebral cortex of the monkey (Macacus). Proc. Roy. Soc , 1924, B 96, 271-291. (Repr. Dtsch. Z. Nervenheilk., 1924, 83: 273-299.)
Dusser de Barenne, J. G., and Sager, O. Über die sensiblen Funktionen des Thalamus opticus der Katze. Untersucht mit der Methode der örtlichen Strychinvergiftung: allgemeine Symptomatologie und funktionelle Lokalisation. Z. ges. Neurol. Psychiat., 1931, 133: 231-272.
Dusser de Barenne, J. G., and McCulloch, W. S. The direct functional interrelation of sensory and optic thalamus. J. Neurophysiol., 1938, 1: 176-186.
Dusser de Barenne, J. G., and McCulloch, W. S. Functional organization in the sensory cortex of the monkey (Macaca mulatta). J. Neurophysiol., 1938, 1: 69-85.
Dusser de Barenne, J. G., McCulloch, W. S., and Ogawa, T. Functional organization in the face-subdivision of the sensory cortex of the monkey (Macaca mulatta). J. Neurophysiol., 1938, 1: 436-441.
Dusser de Barenne, J. G., and McCulloch, W. S. Local stimulatory inactivation within the cerebral cortex, the factor for extinction. Amer. J. Physiol., 1937, 118: 510-524.
Dusser de Barenne, J. G., and McCulloch, W. S. Physiological delimitation of neurones in the central nervous system. Amer. J. Physiol., 1939, 127: 620-628.
Campbell, A. W. Histological studies on the localization of cerebral function. Cambridge University Press, 1905, xx, 360 pp.
Brodmann, K. Neue Ergebnisse über die vergleichende histologische Lokalisation der Grosshirnrinde mit besonderer Berücksichtigung des Stirnhirns. Anat. Anz., 1912, 41 (Suppl.): 157-216.
Bucy, P. C. A comparative cytoarchitectonic study of the motor and premotor areas in the primate cortex. J. comp. Neurol., 1935, 62: 293-311.
Campbell, A. W. Histological studies on the localization of cerebral function. The brain of the gorilla. Rep. Path. Lab. Lunacy Dept., New South Wales Government, 1916, 3: 19-35.
Garol, H. W. Some observations on suppression of electrical activity of areas 4 and 6. Amer. J. Physiol., 1940. 129: 361.
For further research:
Wordcloud: Activity, Animal, Appear, Area, Arm, Arm-Subdivision, Band, Barenne, Boundaries, Brain, Cerebral, Change, Chimpanzee, Column, Cortex, Diagram, Distribution, Dusser, Ecgs, Electrical, Electrodes, Experiments, Extent, Figure, Firing, Following, Found, Functional, Hemisphere, Indicated, Leg, Local, Location, Macaque, Methods, Obtained, Organization, Portion, Postcentral, Posterior, Precentral, Region, Results, Sensory, Spikes, Strychninization, Study, Subdivision, Sulcus, Suppression
Keywords: Chimpanzee, Experiments, Cortex, Organization, Spikes, Report, Distribution, Looms, Paper,
Google Books: http://asclinks.live/rsq0
Google Scholar: http://asclinks.live/qwp3
Jstor: http://asclinks.live/a14c