THE RETICULAR FORMATION COMMAND AND CONTROL SYSTEM12 [208]
W. Kilmer and W.S. McCulloch
##
No animal can fight, go to sleep, run away, and make love all at once. We have therefore listed mutually incompatible modes of vertebrate behavior as follows:
- sleep
- eat
- drink
- fight
- flee
- hunt (prey or fodder)
- search (curiosity)
- urinate
- defecate
- groom
- engage in sex
- lay eggs or give birth
- suckle or hatch
- build nests
Possibly, also:
- migrate
- hibernate
- engage in certain special forms of instinctive behavior
Some may challenge this classification, but the important thing is that there will never be more than, say, 25 modes. An animal is said to be in a mode if the main focus of attention throughout his central nervous system (CNS) is on doing the things of that mode. We hypothesize that the core of the reticular formation (RF) is the structure in vertebrates that commits the animal to one or another mode of behavior (Fig. 1).
The notion of a mode is somewhat subtle, so a little explanation is needed. An animal fleeing in fear may urinate, but he is not in the urination mode. Some of his 13 or more spinal, brain stem, and cerebral urination
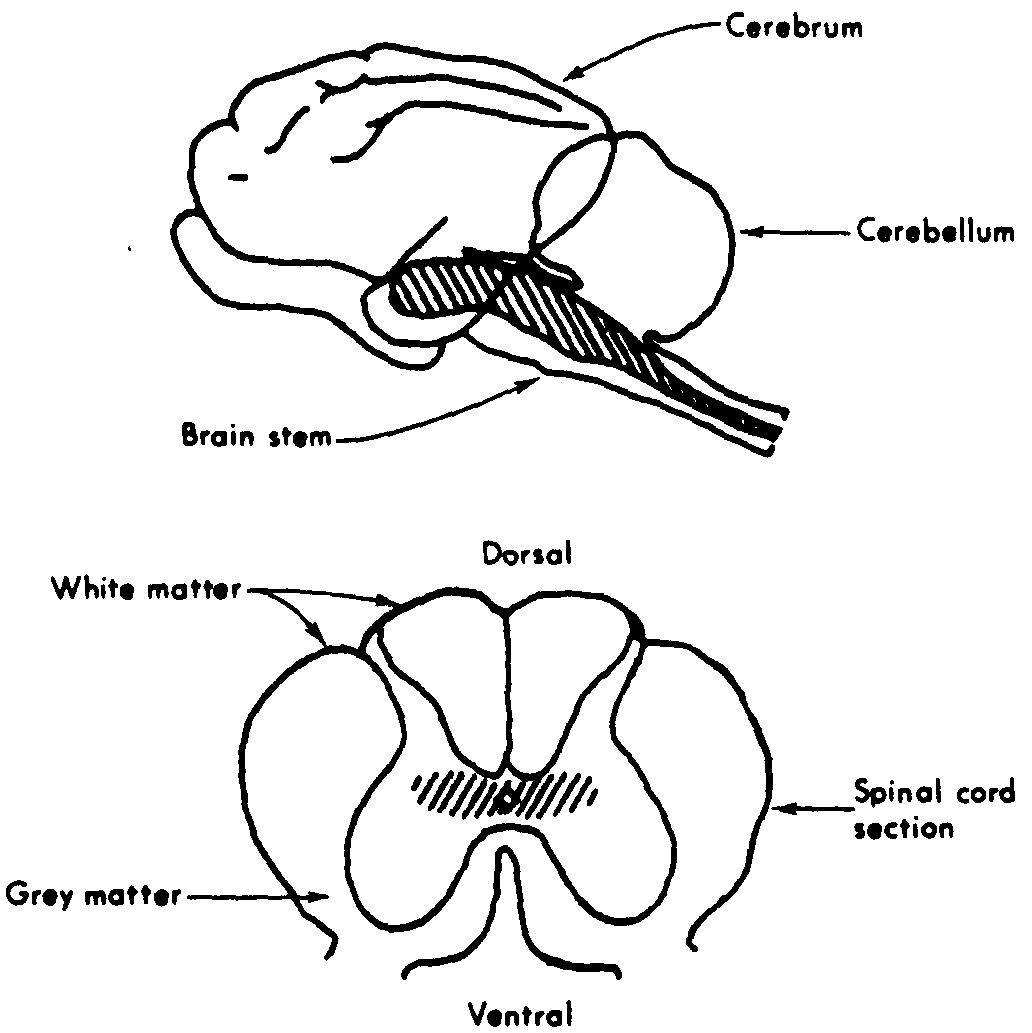
Figure 1. Cat brain and section of spinal cord. RF regions hatched in. The RF extends from the head of the brain stem throughout the core of the spinal cord. (1)
reflexes have been accidentally released in sympathy with his fleeing actions; yet the animal's total attention is directed towards escape. Many birds and fish include components of fighting and fleeing behavior in their courtship rituals when the mode is sexual engagement. Animals can flee in many ways. For example a duck may “flee” by standing still, running, swimming, flying, or using the broken wing trick to lure a predator away from its young in a ground nest. In the last case, it is “fleeing” for itself and also vicariously for its young.
Not all major behavioral patterns are modal. For example, vomiting is not a mode, but an eating trick. This can be seen by noting, for example, that cows use regurgitation as a normal part of their feeding pattern: most birds feed their young by regurgitating; African hunting dogs gulp down prodigious amounts of food after a kill and then return to their burrows and feed the pups and guards that stayed behind by regurgitating; owls often swallow their prey whole and then regurgitate the unwanted bones and hair or feathers; horses almost never regurgitate, nor do reptiles or fish.
If the core of the RF commands the mode of behavior, the RF as a whole serves this command by issuing signals that effect integrated behavior among the various organs and brain parts. An animal's total systemic physiology is known to be very different in sleep, fighting, sexual activity or parturition, and probably also differs considerably among the other modes. This difference is not only autonomic, affective, motoric, and glandular, but perceptual and cognitive as well. In fighting, for example, the visual system sacrifices spatial acuity to gain a slow motion picture of the world, the pain system nearly shuts down, long-range planning is stopped, vocalization is ejaculatory, rage is felt, and movements are stereotyped total action patterns. There is also an almost complete autonomic changeover. RF efferents command all of these things, and participate in the gross control of most of them.
Mode decisions have three important characteristics: (1) The crucial knots of information which prompt them must always get through the sensory filters on which the command system sits. A sleeping mother herring gull is wakened by the new call of its young, but undisturbed by the calls of others. In this case, doubtless cerebral auditory analyzers urge the RF core to wake the gull, and if no other information overwhelmingly conflicts, the gull is wakened. (2) The most important mode decisions are often the most urgent. For example, if a very hungry dolphin is closing in on a fish in the deep ocean and his air supply runs low, the moment-by-moment decision as to whether to continue the hunt or flee to the surface for air, becomes extremely urgent as well as important. Also, the duration required for many fight or flight decisions cannot exceed a fraction of a second—about the duration of Stroud's third of a second moment for humans. Thus, a logically deep serial computer such as man's frontal lobes is ruled out; only a shallow anastomatic one of sufficient scope and economy will suffice. (3) Mode decisions must use a redundancy of potential command in which information constitutes authority. The various regions in a command system that recruit most strongly for a consensus on a mode must be those that have the most exceptional information (compared to a shifting norm) with respect to the ongoing needs and situation of the animal, and vice versa. The other side of this is that no modal command system can allow one of its centers to bully the rest, because the modes and reasons for going in to them are organized as a largely unpredictive heterarchy and not as a predictive hierarchy. Strongly predictive systems are not generally sufficiently open to slight but vitally important information to be reliable mode commanders, especially in unexpected emergencies. Also, most animals at one time and place usually have one set of mode priority relations and at another time and place an entirely different set: a pair of Cichlidae fish chasing each other back and forth out of their respective territories early in a breeding season, but mating and defending a pair-held territory later on (no intervening change in coloration, etc.) is an example of this. State changes in both animals and their environments are generally responsible for such things, of course, but these are best thought of, in the present context, as changes in the kinds of logic performed by brains in different modes and changes in the inputs and intrinsic variables computed by those brains. This only allows a heterarchical ordering of modes, and it requires modal decision systems with a redundancy of potential command.
The oft-cited urgency hierarchy:
- physical support (recovery from fall)
- catching breath
- correcting body temperature and providing shelter, including withdrawal from painful stimuli
- restoring water balance
- reducing hunger
is worked out through intra-modal variations on modal themes.
Modal command systems with potential command redundancy are the least vulnerable to fits and deaths among their components, and this vulnerability decreases as the size of the system increases.
Elsewhere(2) we have justified our RF modal hypothesis in the following ways:
- The RF appears to have direct or monosynaptic two-way connections with every other part of the CNS. Since no other structure has this property, the RF is neuroanatomically best situated to serve as the CNS modal command center. The already demonstrated RF controls over the rest of the forebrain as to consciousness and attention bolster the case.
- Rats with complete transection of the brain stem just posterior to the thalamus and hypothalamus show well integrated (though not sophisticated) behavior except for losses due to blindness, lack of temperature control, and disruption of hormonal regulation. These rats are capable of impoverished versions of most modes of behavior, but are not well tuned in to their surroundings. This suggests that the RF is the modal integrating center, because all the brain such rats have left consists of sensory and motor systems, cerebellum, some basal ganglia, and RF. The cerebellum is considered essentially as an interval clock and cross correlator of relative positions of body parts. It subserves tonic functions, and controls the smoothness and precision of movements. The basal ganglia insure the proper body relationships in posture and motion, especially during well learned sequences of actions. The integrating center in mesencephalic rats thus, is probably the RF.
- Local electrical and chemical stimulation of intact animals in descending pathways just anterior to the RF has switched them into sleeping, eating, drinking, fighting, fleeing, urinating, searching, grooming, sexual, and nest building modes. Apparently any mode can be entered in this way under the proper physiological conditions. The important thing is that such stimulated behavior of animals is specific as to mode but dependent upon social relationships, memories, and environmental cues for details. Thus, the mode is what is activated.
- The main effects of debilitating temporary or permanent lesions in the RF are of two types: firstly, formerly integrated behavior disintegrates, as when the sleep of the brain and the body get out of step, or when the physiological concomitants of paradoxical sleep dissociate; secondly, switching from mode to mode is pathological, as when cats awake frequently out of deep sleep and pass immediately into extreme rage or fright, or when akinetic mutes cannot get out of the sleep and resting mode.
- It is becoming increasingly clear that an animal's feeling states and drives to action originate mostly in the periventricular system of the brain stem and hypothalamus. The RF core neurons which constitute most of this region are morphologically identical from shark to man. Since the lower in phylogeny the animal, the more sharply modal its behavior, the core of the RF would seem to be intrinsically organized for operation in one or another stable mode. Rioch(3) noted that in mammals the stereotyped behavior patterns mediated by the paleocortex and the highly differential functions performed by the neocortex could probably not have evolved unless the underlying brain stem integrative patterns were stable and modal.
The main plastic behavior found in the RF relates to development (almost entirely prenatal), habituation, and classical and avoidance conditioning on modes and autonomic responses.
We conceive, then, that the RF core commits an organism to a mode with a number of relatively undifferentiated neurons which is estimated to be less than the number of afferent peripheral neurons, or bipolars, and greater than the number of efferent peripheral neurons, or motoneurons. This number is about three quarters of a million in frog and perhaps two million in man. Jointly, these sample all signals from the bipolars and all signals ascending or descending in the neuraxis, including those from all other parts of the nervous system, each neuron receiving, perhaps, a thousand of these, selected almost at random. The axon of each divides into an ascending and a descending branch, often having, in addition, a collateral that ends on or among its dendrites and those of its neighbors. By these axons, the reticular core not only controls all other parts of the nervous system but many other neurons of its own system (Figs. 2 and 3). Our primary concern is with its intrinsic organization. How, in a fraction of a second, can a million or more computing components reach a workable consensus as to the proper mode of total commitment? This is our problem.
Since neurons are nonlinear oscillators, we would have liked, for verisimilitude, to have used the mathematics of oscillator coupling, but it was inadequate because it dealt only with steady states and not transient computations. Since the reticular core is an iterated net of sorts, we would also

Figure 2. RF dendritic organization. (a) RF cross section, showing dendritic organization on left, and input organization on right. (b) Sagittal section through the lower half of the brain stem of a 10-day-old rat. Collaterals from the pyramidal tract (Tr. pyr.) and from a single reticular cell axon illustrate the tendency toward organization of the afferent terminals in planes approximately perpendicular to the long axis of the stem. The organization of reticular dendrites appears to parallel these terminals—in contrast to the dendrite organization of the adjacent hypoglossal (XII) nucleus—so that the reticular core might be considered as a series of neuropil segments (insert diagram).(4)
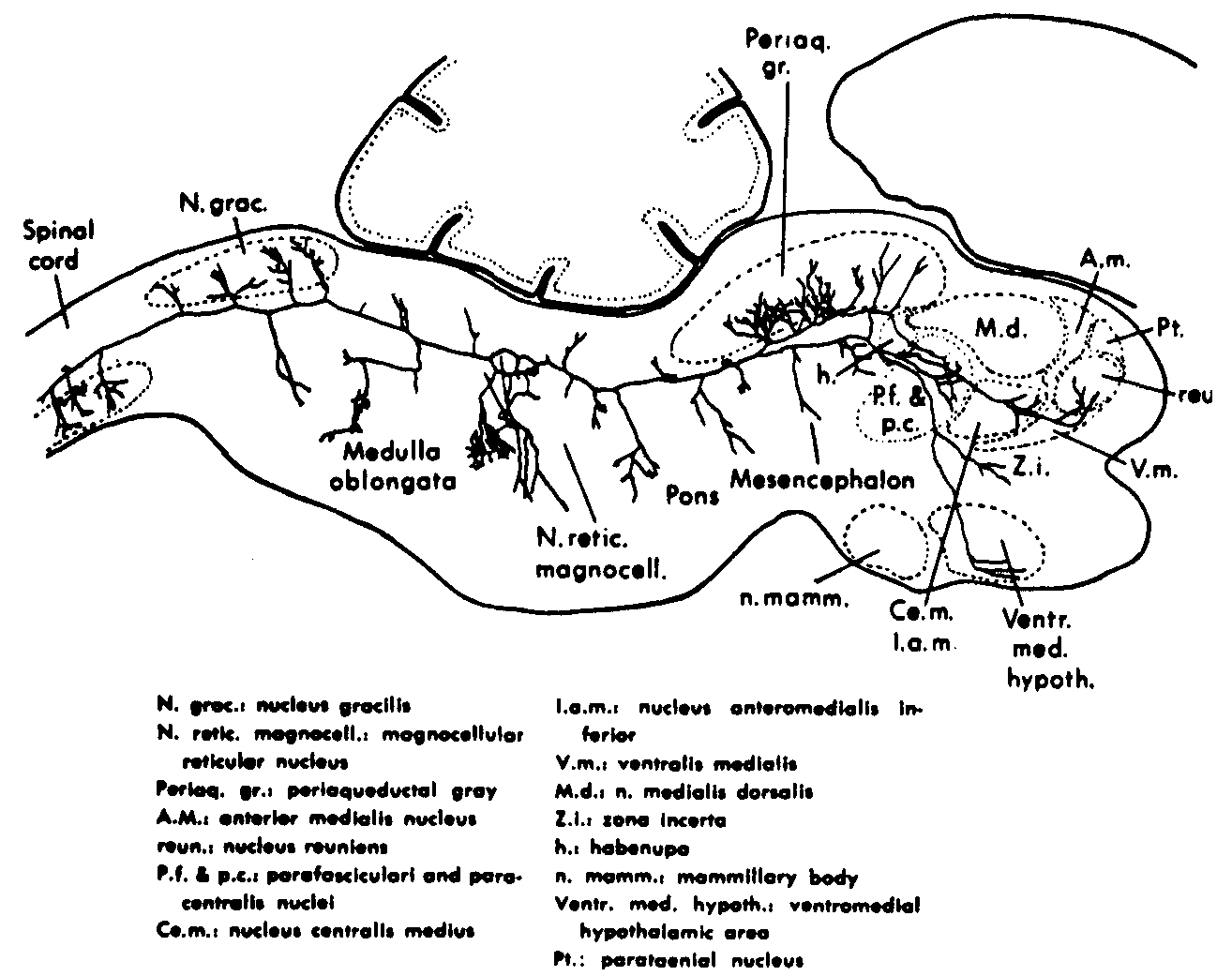
Figure 3. Sagittal section of 2-day-old rat, showing a single large reticular cell of the magnocellular nucleus. It emits an axon which bifurcates into a caudal and rostral segment. The caudal segment gives off many collaterals to the adjacent reticular formation, to the nucleus gracilis, and to the ventral nuclei of the spinal cord. The rostrally running segment gives off collaterals to the reticular formation and to the periaqueductal gray substance, and then appears to supply the parafascicular and paracentral nuclei, the centromedian and interanteromedian nuclei, the reuniens nucleus, and the ventromedian hypothalamus, as well as the zona incerta.(4)
have liked to use iterated net theory, but every question we thought worth asking proved recursively insoluble.
The only remaining course was through the art of combinations which multiply rapidly as the number of related items increases by a single step. Therefore, we decided to deal with small numbers of possible inputs and computing modules, using modules that were loosely coupled. Five modules did not give us room for a probabilistic distribution of functions of the input, but at six modules the computation got out of hand. So we were forced to use computer simulation. Still there was too little room, and we finally had to go to twelve modules. We decided to settle, for the time being, on four incompatible modes of behavior and ended with our present S-RETIC model (Fig. 4).
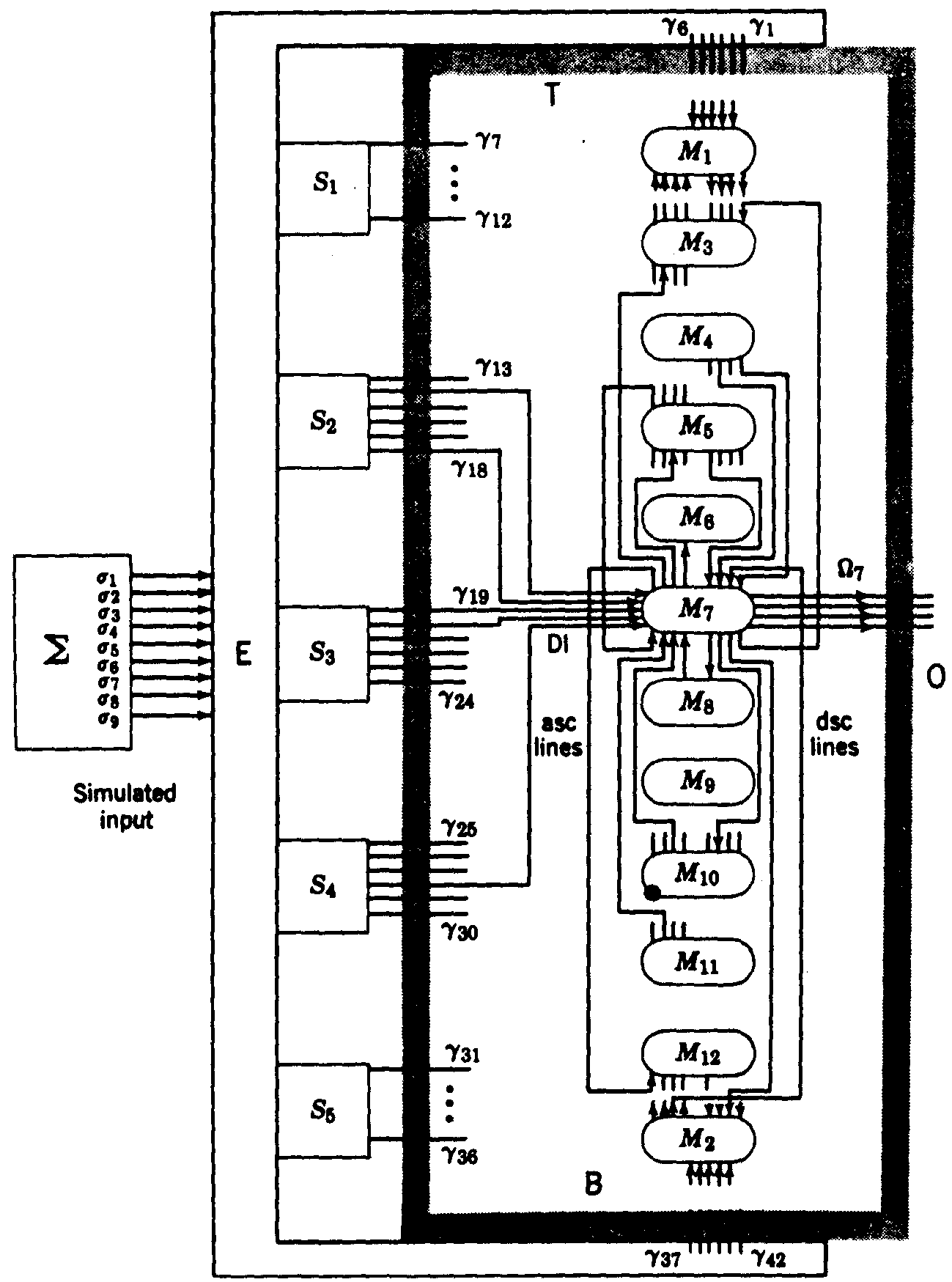
Figure 4. S-RETIC Simulation model. Connections are shown only about M7. Each M1 is a caricature of a poker chip region, or neuropil segment, in Fig. 2b. The S1 are input systems and the Ω1 comprise S-RETIC's output. E generates S1 values by computing functions of the irredundant set of binary variables out of Σ. S-RETIC is said to have converged on a mode if more than half of its M1indicate that mode with probability (preference) greater than .5.(1)
Each module of S-RETIC, pictured as a neuronal computing chip, receives a few channels which inform it well of a few and poorly of most of the functions computed on the inputs at other modular sites. Each module, then, computes the best guess it can about the probability of the proper mode and informs a random group of other modules of what it thinks the odds are on the mode to be selected. It also receives the guesses of some other modules chosen at random. It then forms a new opinion and repeats the process. Clearly, a module that regards all modes as equally likely to be right should have little weight, whereas one that has a strong preference should be heard because it probably has the necessary evidence to warrant it. Hence, the value of a mode preference should be rated in a nonlinear manner representing the intensity of preference. Only trial and error on the computer could tell us what the nonlinearities should be to always ensure convergence to a workable consensus among the modules as to proper mode within a small number of interchanges of opinion, for instance less than 25. (In the reticular core this would take less than a third of a second.)
Note that unless there is some strong reason for changing its opinion, a module gives less and less attention to its more extreme inputs as a mode persists. This, if left unmodified, would have prevented S-RETIC from being too distractible, but would have made it pigheaded and allowed an entrenched mode to persist even when some modules had significant new inputs that demanded a change of mode. We remedied the situation by arranging for each module to uncouple from its fellows upon the presentation of a new input to a degree that increases with both the significance of the new signal received and the degree to which the old mode had been entrenched. The degree of decoupling cannot be simply proportional to either or both. The proper nonlinear function could be found only by trial and error. Fortunately, here again the exact form of the nonlinearity need not be optimal, provided it is of the right kind and never too great. In fact, there may be no optimum function for all real contingencies.
The continual reverberation of signals among the modules of S-RETIC constitutes a distributed memory which is just sufficient to form conditioned reactions, provided nothing intervenes between the conditional and unconditional stimuli. Its time-binding ability can be sufficiently increased to yield conditioning with short intervening events by introducing short delay lines. (For longer delays we could use shift registers, and for these, Jose da Fonseca,(5) has given us the algorithm for making the shortest nonlinear ones to produce a given sequence.)
We should add that our model does what it is supposed to do, that it has been enlarged to simulate habituation, classical conditioning and extinction, and avoidance conditioning. It also can be enlarged to accommodate a selection of one of a dozen or more modes, and its number of modules can be increased indefinitely without markedly increasing the duration of “conversation” among the modules to reach consensus. In so doing, it becomes more reliable, not less, because of its redundancy of potential command.
Our simulation of S-RETIC and its successors was not devised to be the most economical, but to be thoroughly transparent and easily modified. We are indebted to Richard Warren for the appropriate philosophy and to Jay Blum for the programming. They have made it possible for us to think, not merely about, but with the computer, in a kind of dialogue in which each helps the other to enrich and revise his processes. Without that dialogue we would never have been able to make or explain a model that does in a logical sense what we believe the vertebrate reticular core does in a phenomenal sense. The reticular core is not supposed to usurp the role of the forebrain in perception in forming propositions and in long-range planning, for these are inductive processes. It is not to supplant the executive organs of the rest of the nervous system in programmed and controlled activities, for such logic as they enjoy is basically deductive. They know that they have a case to be handled under a given rule and proceed to perform their proper acts.
The reticular core cannot invent new modes and it cannot perform what it commands. Its logic is abductive, the Apagōgē of Aristotle. Given the rules, it must, when confronted with the fact, guess which rule applies. This, S-RETIC does; and, since it is a logical model, it is not restricted as is its nervous counterpart, but is applicable to the command problem of a battle fleet, the deliberation of a grand jury, or the patient classification problem of a diagnostic clinic. Greater verisimilitude to the vertebrate reticular core, making it better for devising experiments, will only limit its scope.
It is one thing to have a working model, and quite another to have a clean theory. For the first inkling of that theory we are indebted to Roberto Moreno-Diaz.(6) The theory grew out of Peirce's(7) logic of relations, and employs what he has called “functional state transition matrices.” It can handle the behavior of neuronal nets, whether they are deterministic or probabilistic, or depend for their transitions upon continuous or discrete variables. Hopefully, we will have more to say on this in years to come.
In the meantime, our simulation work has led us to a new outlook on some RF experiments. The S-RETIC model shows that a nonlinear redundancy of potential command, and extensive decoupling of modules upon significant input changes, is sufficient for the operation of a command computer. The question arises as to what evidence there is for decoupling, or inhibitory quenching, of circulating signals in the RF core upon the appearance of a mode changing effect in the animal. Now, the Scheibels(8) have found that most RF neurons apparently undergo cyclic shifts in their receptive field sensitivities. By investigating the entrainment of such cycles, one might shed some light on the decoupling question. If cycling entrainment exists, but only as a statistical coherence that changes with the behavioral mode of an animal, on-line data processing from electrodes in animals that move freely in fixed circadian environments might be revealing. Such experiments would pose grave technical problems but, if achieved, could give as a by-product much better data on the probabilistic character of mode transitions than is now available. One wonders if these transitions are in general governed by the simple time-varying Markov probabilities that Calhoun's(9) data on rat sleep suggests. (In non-sleep modes, of course, environment cue schedules would have to be taken into account.)
Another question that our recent modeling work has raised is whether or not a RF response to a new input can be broken up into a short phase of novelty-detection superimposed on a long-period of coupled-circuit combinational computing. If so, the main significance of this would be suggested by our new H-RETIC model, which is now in a late design phase.
Footnotes
References
Kilmer, W., McCulloch, W. and Blum, J. (1969a). Embodiment of a plastic concept of the reticular formation. Proc. Symposium on Biocybernetics of the Central Nervous System. Boston: Little and Brown.
Kilmer, W. and McCulloch, W. (1969b). The biology of the reticular formation, to be published as a Michigan State University Division of Engineering Research Report under Grant No. AF-AFOSR-67-1023B.
Rioch, D. M. (1954) In E. D. Adrian, Brain mechanisms and consciousness. (Eds.) et at. London: Oxford University Press.
Scheibei, M. E. and Scheibel, A. B. (1958). Structural substrates for integrative patterns in the brain stem reticular core. In H. H. Jasper et al. (eds.). Reticular formation of the brain. Boston: Little and Brown.
Fonseca, J. de (1967). Synthesis and linearization of nonlinear feedback shift registers—basis of a model of memory. M.I.T., Quarterly Progress Report, 86: 355.
Moreno-Diaz, R. (1969). On a calculus of relations. Proc. Symposium on Biocybernetics of the Central Nervous System. Boston: Little and Brown.
Peirce, C. S. (1933). Collected papers of C. S. Peirce. Vol. III. Exact logic. Cambridge, Mass.: Harvard University Press.
Scheibel, M. E. and Scheibel, A. B. (1967). Anatomical basis of attention mechanisms in vertebrate brains. In G. Quarton et al. (eds.), The neurosciences, a study program. New York: Rockefeller University Press.
Calhoun, J. B. (1968). Private correspondence.
For further research:
Wordcloud: Animal, Behavior, Brain, Changes, Command, Computer, Conditioning, Control, Core, Decisions, Different, Example, Fighting, Figure, Fleeing, Formation, Functions, Give, Important, Increases, Information, Input, Integrated, Logic, Modal, Mode, Model, Modules, Nervous, Neurons, Nonlinear, Number, Organization, Patterns, Probably, Processes, Proper, Rats, Relations, Reticular, S-Retic, Signals, Sleep, State, Stem, Sufficient, System, Vertebrate, Work
Keywords: Command, Some, Formation, Behavior, Stem, Thing, System, Mode, Psychology, Animal,
Google Books: http://asclinks.live/65p2
Google Scholar: http://asclinks.live/6lug
Jstor: http://asclinks.live/1v3s