TWO REMARKS ON THE VISUAL SYSTEM OF THE FROG 12 [155]
J.Y. Lettvin, H.R. Maturana, W.H. Pitts and W.S. McCulloch
Part I. Form-Function Relations in the Retina
In two earlier papers(1, 2) we described the operations on the visual image that were found in the fibers of the optic nerve in Rana pipiens. We remarked then that these operations seemed related to the anatomy of retinal cells. This note will show what kind of form-function relations we think hold. It is provisional, a program rather than a solution.
If we restrict ourselves to form perception such as applies to silhouettes, then we can say what qualities of the visual image are displayed in the output of the frog's retina. Neither color nor shading enters into the account, which is thereby incomplete. Nevertheless under that restriction we can account for almost all the fibers in the optic nerve and show that there are four operations on the image, each carried by a different population of axons. Each operation is invariant under change of average intensity of light by which a given silhouette-background combination is seen. Each operation is also uniformly distributed over the retina. There is, in addition, one group of fibers that respond to average illumination only, and we can say little about their distribution.
Now the anatomy of the frog's retina shows, according to Ramon y Cajal,(3) five types of ganglion cells whose axons make up the optic nerve. These types differ from each other by the shapes of their dendritic arbors—in the same way that species of trees differ when seen in outline. Furthermore the frog's retina has the same transverse structure from place to place along itself, there being no fovea, and the uniform distribution of the operations is matched by the symmetrical anatomy of the retina. Recause the number of functions we discriminate in the optic nerve matches the number of ganglion-cell types, it is natural to ask whether one can assign particular shapes to particular functions.
The material being combined arises from our own experiments and from Ramon y Cajal's 1894 treatise on the retina (which has been amplified by one of us, HRM).
Synopsis of anatomy
Rods and cones (Figs. 1, 2, 3). There are two sorts of rod and one kind of cone in the frog's retina. The red rods are most numerous, closely seconded by the cones. The green rods are relatively scarce. All the photoreceptors are uniformly distributed over the retina. The cell bodies of the rods and cones lie in the outer granular layer internal to the outer limiting membrane. Their “feet” issue into the outer plexiform layer. The feet of the red rods end in the outer zone of that layer, those of the cones in the middle zone, and those of the green rods in the inner zone.
Bipolar cells (Figs. 1, 2, 3). There are essentially three sorts of bipolar cells; their cell bodies lie in the middle granular layer. They are to be distinguished not so much on the basis of size as of connectivity, although the first two kinds are large and the third small. Type I has a dendrite that spreads out in the middle zone of the outer plexiform layer and connects widely and exclusively with the cones, its axon bushes out widely in the outer zone of the inner plexiform layer. Type II has a dendrite that also branches in the middle zone of the outer plexiform layer, but in a very constricted way, and sends processes into the outer zone. (Ramon y Cajal thinks they connect exclusively with the red rods but is not sure.) Its axon arborizes at several levels of the inner plexiform layer. Type III has a dendrite that connects also over a constricted field, but seemingly with different types of photoreceptors, and in addition sends a process, the Landolt club, up between the somata of the photoreceptors to bulge out at the level of the outer limiting membrane. Its axon penetrates deepest in the inner plexiform layer but emits planar arbors at several of the outer levels.
Horizontal and amacrine cells (Figs. 1, 2). There are two sorts of lateral connectives in the retina. The horizontal cells have their bodies at the outer margin of the middle granular layer, and both dendrites and axons arborize in the outer plexiform layer. The amacrine cells have their bodies at the inner margin of the middle granular layer and emit wide-spreading processes into the inner plexiform layer. Different cells spread out in different levels, and there are
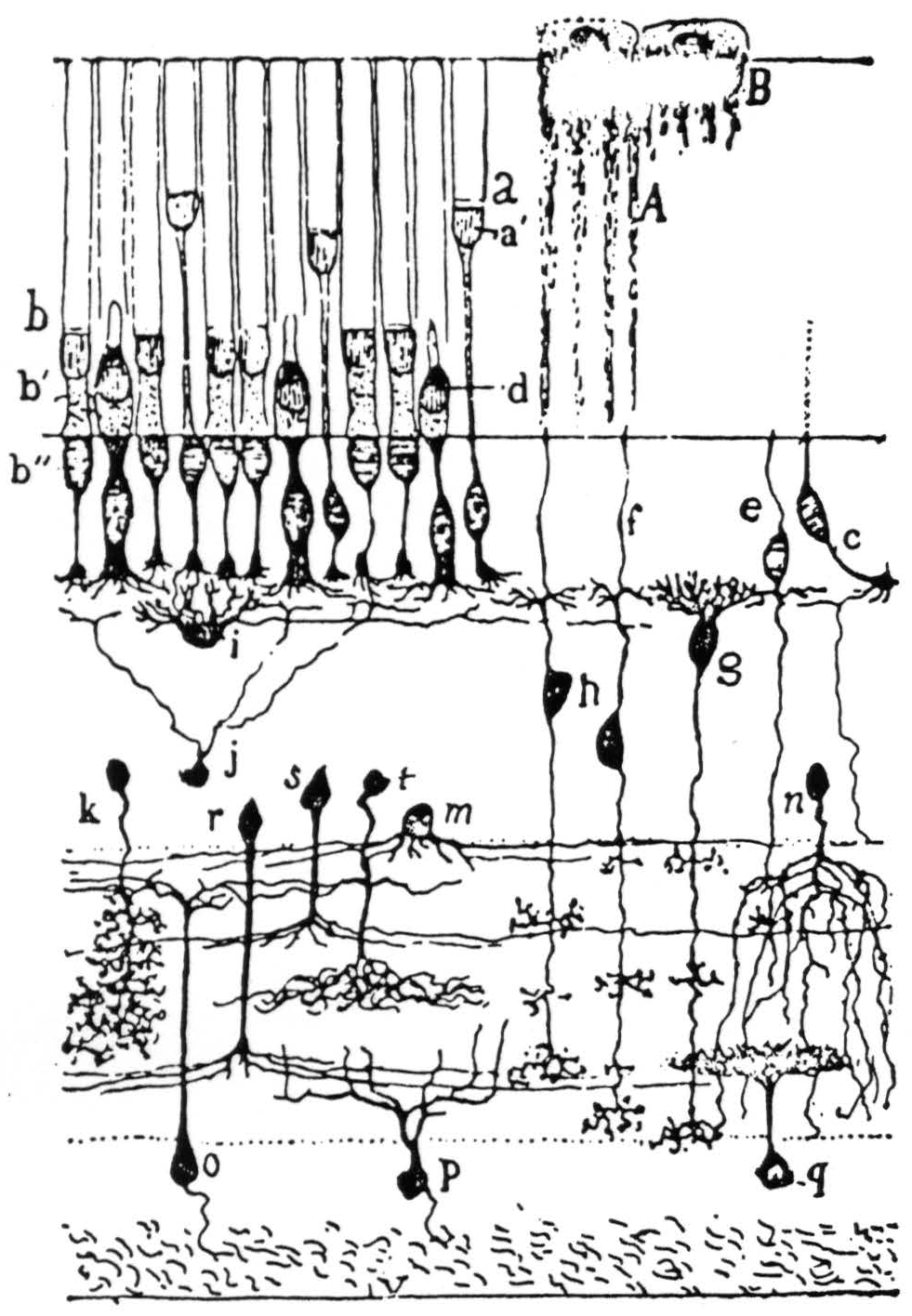
Figure 1. The retina of frog in Golgi stain—highly schematic but showing spatial arrangement: g and h are bipolar cells; i is a horizontal cell; k, r, s, t, and m are amacrines; o, p, and q are ganglion cells. This is the bad off-hand diagram used for illustration in Ramon y Cajal's Histologie du Système Nerveux (Paris: Maloine, 1909-1911). The actual picture of frog's retina in exploded diagram is shown in Fig. 2.
several kinds of arbor—thin- and thick-branched, dense and rarefied. Amacrine cells do not have axons in the anatomical sense. No more is known about them, but they are commonly accepted as nervous elements.
Ganglion cells (Figs. 2, 4). The axons of these cells make up the optic nerve. There are half a million of them in the frog, body to body in a single sheet, the inner granular layer. The count tallies
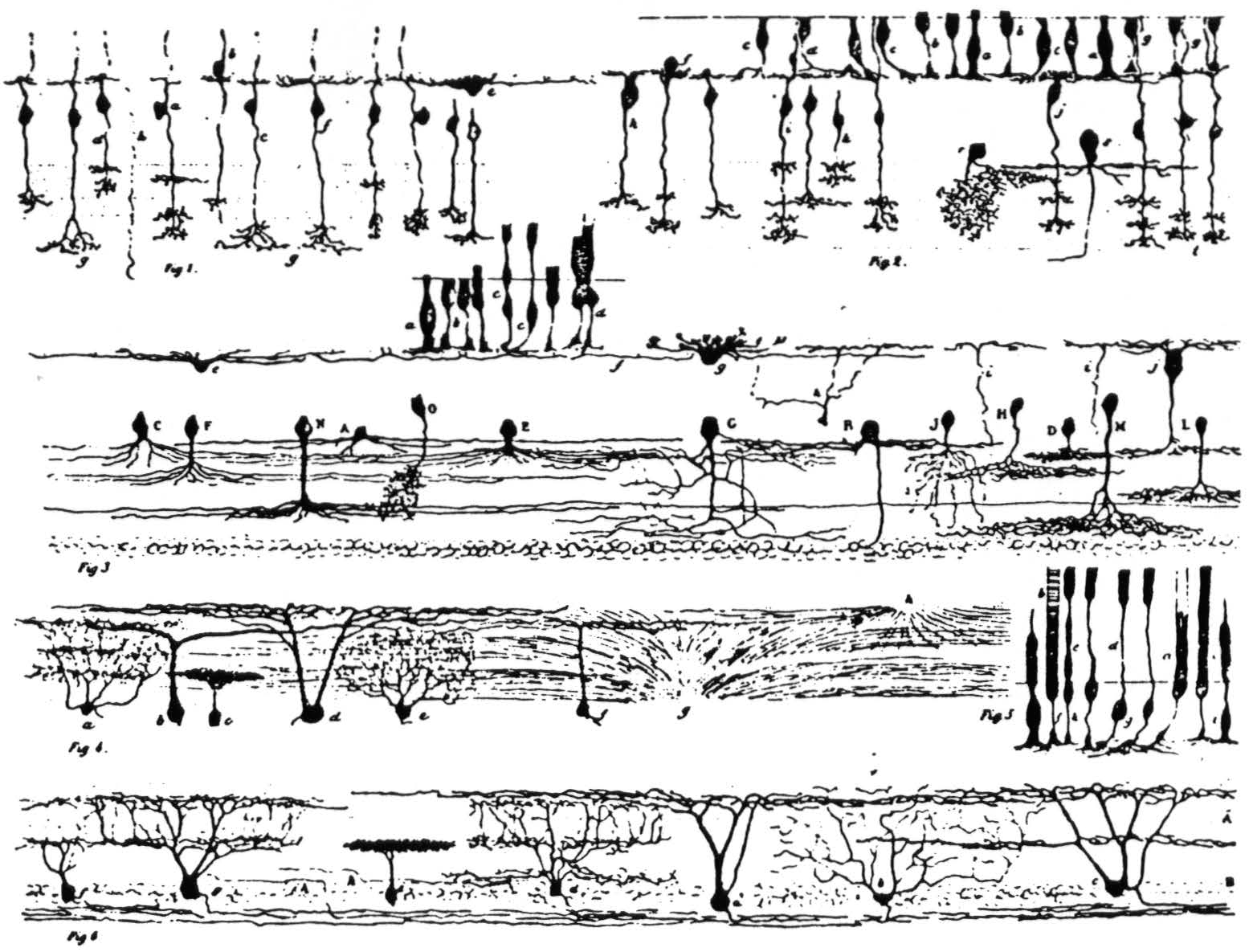
Figure 2. Ramon y Cajal's detailed illustration of the frog retina from the 1894 study. It consists of six subfigures, as follows: (Fig. 1) Various bipolar cells; one horizontal cell is labeled e. (Fig. 2) Bipolars and rods and cones. (Fig. 3) a, b, c, d are rods and cones, g is a horizontal cell, j is type I bipolar in our classification; the cells with capital letters are amacrines. (Fig. 4) Some ganglion cells. (Fig. 5) Rods and cones. (Fig. 6) Ganglion cells: e shows the single-layer constricted dendritic spread; a is the single-layer extended spread; c is the many-level II distribution of dendrites; d and g are the many-level E distributions; b has a diffuse dendritic tree.
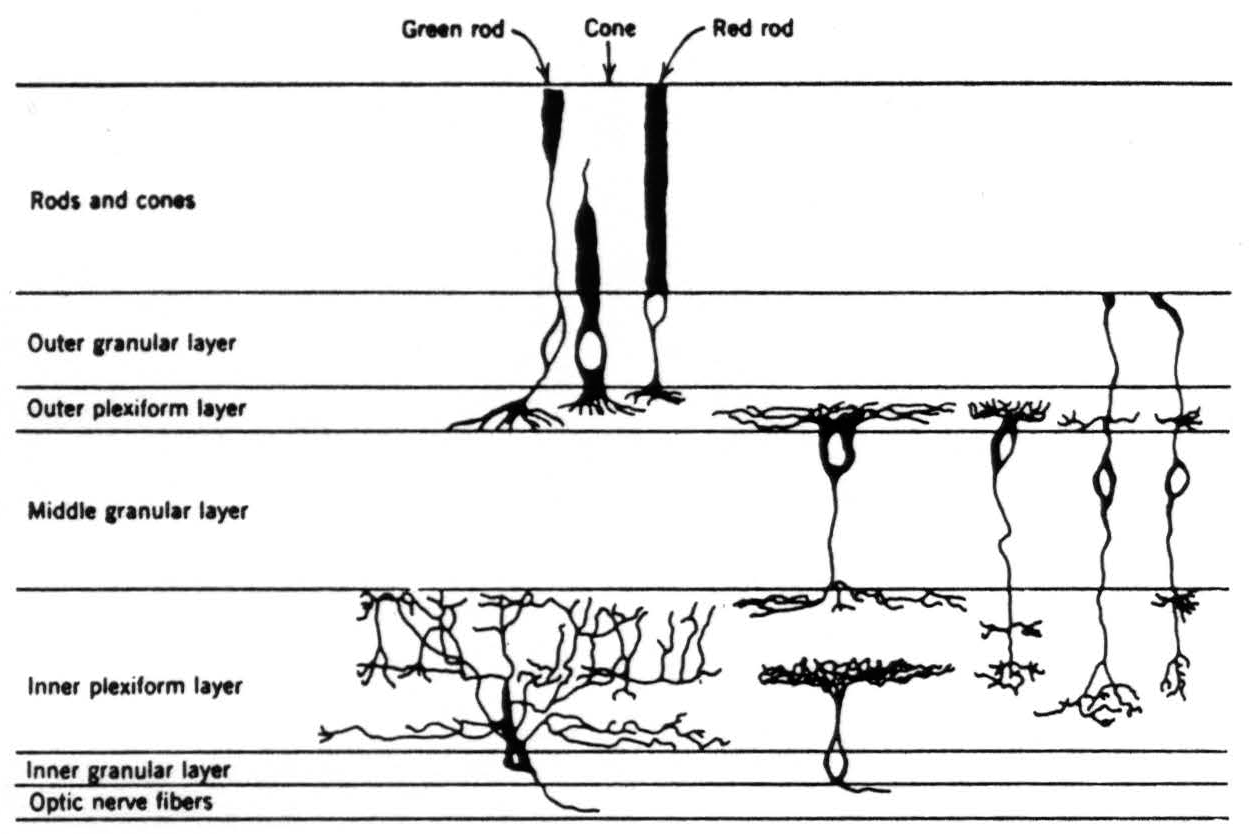
Figure 3. Diagram of the retina excerpted from Fig. 2 to show the schema we are using in this paper.
with the number of fibers in the nerve.(4) Many of these cells have been erroneously thought to be glial because of the great inaccuracy of former estimates of the number of fibers in an optic nerve.(4) One can distinguish five types on the basis of shape and size of the dendritic trees, as revealed by Golgi stains (Fig. 4).
- One-level constricted field (Fig. 4; 6e in Fig. 2). These are the smallest of the ganglion cells; their axons are difficult to stain or see. The major dendrites extend only to the inner levels of the inner plexiform layer and there spread out in dense and constricted planar bush.
- One-level broad field (Fig. 4; 6a in Fig. 2). These are the largest of the ganglion cells. The major dendrites extend to the outer levels of the inner plexiform layer and there branch widely over a considerable area.
- Many-level H distribution (Fig. 4; 6c in Fig. 2). These are next to the largest of the ganglion cells. The major dendrites extend to the outer levels of the inner plexiform layer, as do those described in (2) above. However, they emit two widely spread arbors, one in the inner levels and one in the outer levels.
- Many-level E distribution (Fig. 4; 6d and 6g in Fig. 2). These are next to the smallest ganglion cells. The major dendrites extend only to the inner levels of the inner plexiform layer and there branch out in planar fashion. However, each branch emits twigs all along its course, and some extend into the outer levels of the inner plexiform layer, whereas others remain in the inner levels.
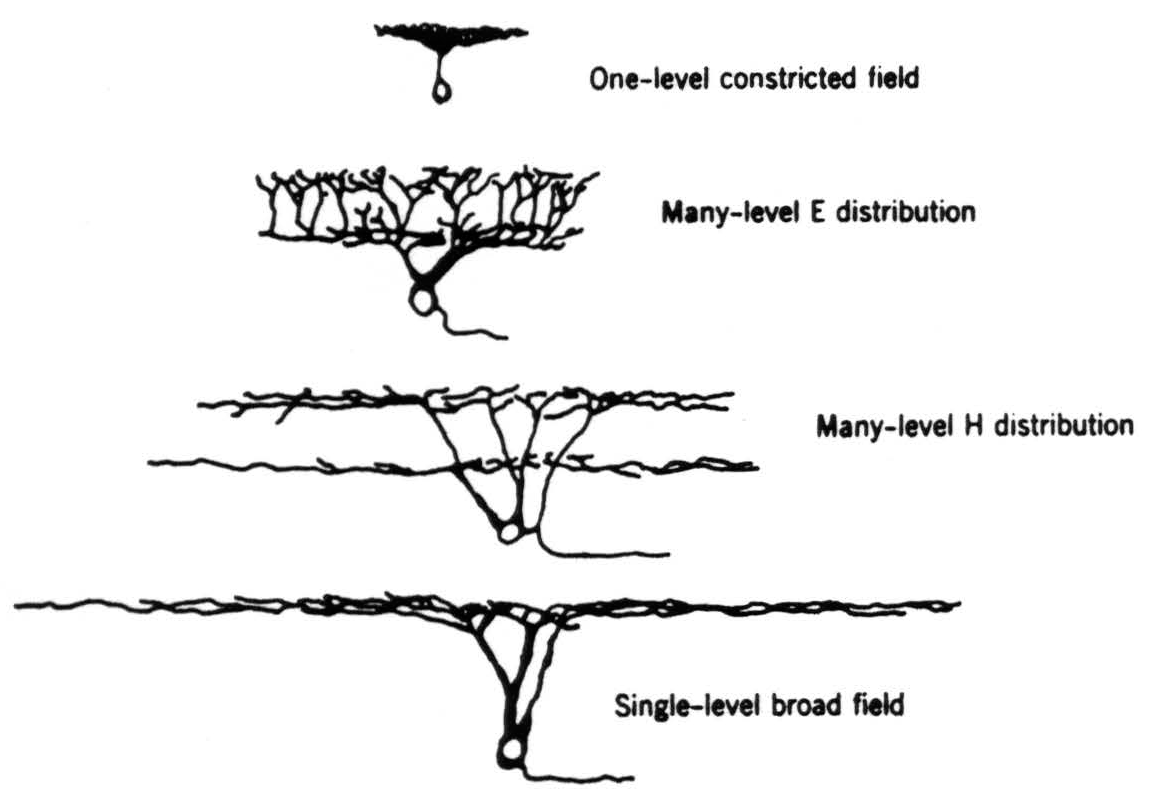
Figure 4. The four types of ganglion cell (exclusive of the diffuse dendritic tree) compared for shape and relative size on the same scale.
- Diffuse trees (Fig. 3; 6b in Fig. 2). There are several sizes of these cells. The dendrites branch helter-skelter all over the inner plexiform layer and show no planar arrangement such as occurs in the other four kinds of cell.
You will notice that the dendritic arrangements shown in the figure suggest that there are two major subdivisions of the inner plexiform layer, although undoubtedly each subdivision can be divided into several levels.
Synopsis of physiology
As is apparent from the anatomy, any ganglion cell in the frog is connected via the bipolar cells at least to several hundred, or several thousand, photoreceptors. This connectivity is reflected in the observation by Hartline(5) that any optic nerve fiber responds to visual events over an area of several degrees in diameter on the retina. That area is usually centered over the cell body; it is called the receptive field. We found four distinct types of receptive field for fibers in the optic nerve and one kind whose boundaries we could not measure. The types of receptive field are given below, together with the properties of the axons to which they belong.
Perhaps we had better say a word about our experimental procedure, although a full account appears in our other two papers. Our stimuli consisted of silhouettes of different size and shape, cut out of matte white, gray, or black paper and seen against matte white, gray, or black backgrounds. The targets were mounted on soft iron washers and moved against the background by means of magnets. They were viewed by reflected light, and the intensity of that light could be varied. Response of a nerve fiber was measured roughly by frequency and duration of firing. We took no records except those needed to illustrate our papers. Our question was not how great the response was to one or another manipulation, but rather which visual events produced greatest response and which produced least, and what aspect of the image could be varied without changing the response. We dealt with our own listening to the patterns of nerve spikes as the measure of extremes, just as one does with an a-c bridge. (Early in our work we had found that taking records hindered rather than helped this kind of research by leading to premature standardizing of method.)
The groups we discovered are as described in the following pages.
Unmyelinated fibers
These conduct at velocities of 20 to 50 centimeters per second. They make up 97 per cent of the population in the optic nerve.(4)
Group I, the boundary detectors (Fig. 5). These fibers have receptive fields, 2 to 4 degrees in diameter. They respond to any boundary between two grays in the receptive field, provided it is sharp. Sharpness of boundary rather than degree of contrast seems to be what is measured. The response is enhanced if the boundary is moved and is unchanged if the illumination of the particular contrast is altered over a very wide range. If no boundary exists in the field, no response can be got from change of lighting, however sharp that change. If a boundary is brought into the receptive field in total darkness and the light is switched on, a continuing response occurs after a short initial delay. Such axons are the “on” fibers of Hartline—a small well-focused spot of light is defined by a sharp boundary.
Group II, the movement-gated, dark convex boundary detectors (Fig. 6). These fibers have receptive fields of 3 to 5 degrees. They too respond only to sharp boundaries between two grays, but only if
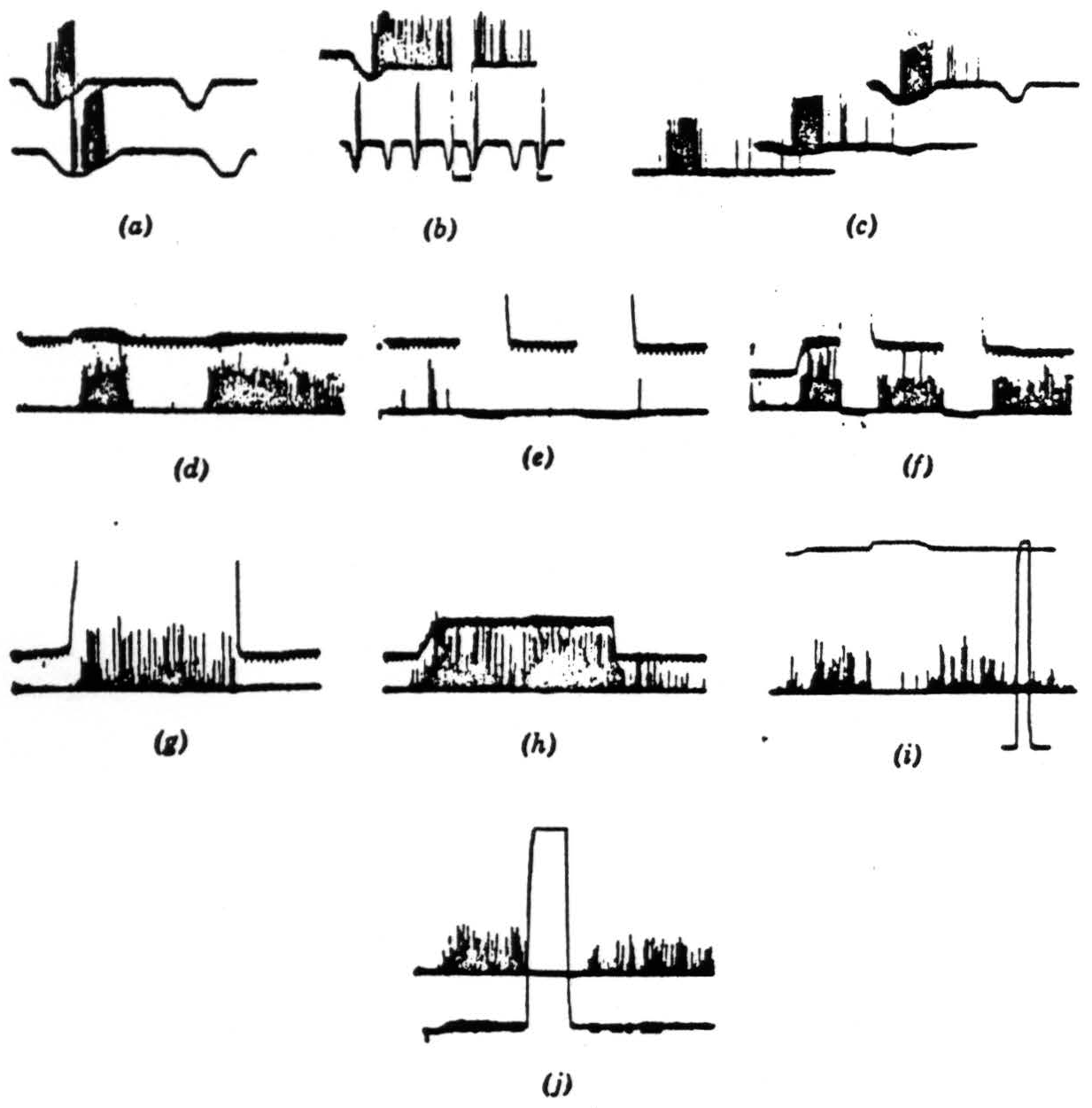
Figure 5. The records were all taken directly with a Polaroid camera. The spikes are clipped at the lower end. just above the noise, and brightened on the screen. Occasional spikes have been intensified by hand for purposes of reproduction. The resolution is not good, but we think the responses are not ambiguous. Our alternate method of recording is by means of a device that displays the logarithm of the pulse interval of the signals through a pulse-height pick-off. However, such records would take too much explanation and would not add much to the substance of the present paper. Operation 1. Contrast detectors. (a) This record is from a single fiber in the optic nerve. The base line is the output of a photocell watching a somewhat larger area than the receptive field of the fiber. Darkening is given by downward deflection. The response is seen with the noise clipped off. The fiber discharges to movement of the edge of a 7-degree black disc passed in one direction but not in the reverse movement. Time marks, 20 per second. (b) The same fiber continues to respond when the edge of the disc stops in its receptive field. The response disappears when the illumination is turned off and reappears when it is turned on. The lower line shows again the asymmetry of the response to a faster movement. Time marks, 20 per second. (c) The same fiber is stimulated to show an asymmetrical response to the 7-degree black object moved in one direction and then in the reverse direction, and the stimuli are repeated tinder a little less than a 3-decade range of illumination in two steps. The bottom record is in extremely dim light, the top in very bright light. Time marks, 20 per second. (d) In the lower line, a group of endings from similar optic nerve fibers is shown recorded from the first layer in the tectum. A black disc 1 degree in diameter is moved first through the field and then into the field and stopped. In the upper line the receptive field is watched by a photomultiplier (see text), and darkening is given by upward deflection. Time marks, 5 per second for all tectal records. (e) Turning off and on of general illumination has no effect on these fibers. (f) A 3-degree black disc is moved into the field and stopped. The response continues until the lights are turned off but reappears when the lights are turned on. These fibers are nonerasable. (g) A large black square is moved into the field and stopped. The response to the edge continues so long as the edge is in the field. (h) The 3-degree disc is again moved into the field and stopped. When it leaves, there is a slight after-discharge, (i) A 1-degree object is moved into the field and stopped; the light is turned off, then on, and the response comes back. The light is approximately 300 times dimmer than in the next frame. Full on and off are given in the rectangular calibration at the right. (j) The same procedure as in (i) under very bright light. The return of response after reintroduction of the light seems more prolonged, but only because in (i) the edge was not stopped in optimal position.
that boundary is curved, the darker area being convex, and is moved or has moved. That is to say, this fiber does not respond to changes of lighting, however sharp, if no boundary exists in the field, and it also does not respond to a sharp, straight boundary, moving or stationary, in the receptive field. It responds to a net curvature of the boundary if the darker gray is convex and the movement centripetal, or if the lighter gray is convex but is sufficiently relieved against the background to have a sharp shadow at its edge. It does not seem to matter how much darker the target is than the background beyond a certain very small contrast. When the curvature is so great that the target is a small area, about half the diameter of the receptive field, then, if the target is moved centripetally and stopped before the center of the receptive field, it sets up a long-lasting discharge. This discharge, however, is erased by a transient dimming of the light or by a shadow passed over the receptive field, and it will not recur until the boundary is moved again even very slightly. Here, also, small movements of the boundary enhance the prolonged discharge transiently. Again, the responses are invariant over a wide range of illumination, roughly that between dim twilight and bright noon. There are other remarkable aspects of this class of fibers, and they are discussed in our other two papers. Suffice it to say that they have not been seen before because they do not respond to spots of light whose boundaries are convex with respect to the darker gray and which are not moved.
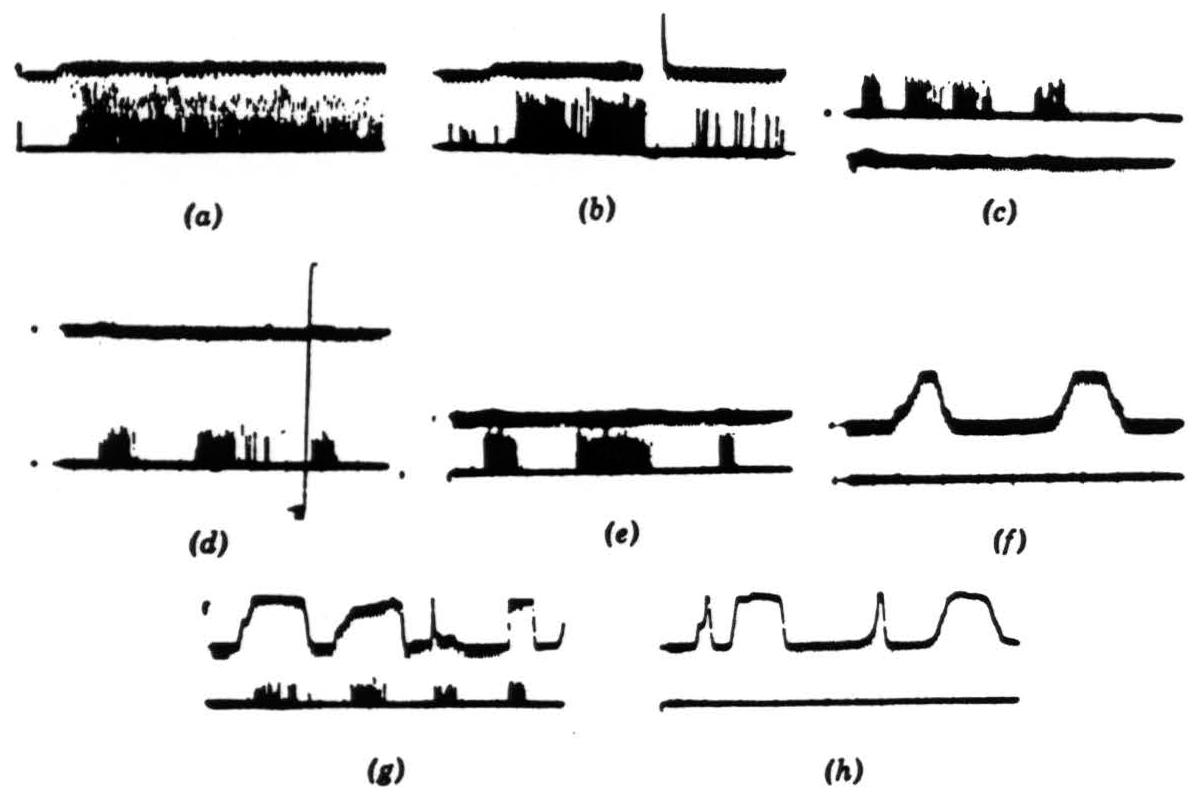
Figure 6. Operation 2. Convexity detectors. The photomultiplier is used, and darkening is an upward deflection. (a) These records are all from the second layer of endings in the tectum. In the first picture, a 1-degree black disc is imported into the receptive field and left there. (b) The same event occurs as in (a), but now the light is turned off and then on again. The response is much diminished and in the longer record vanishes. These fibers are erasable. (c) The 1-degree disc is passed through the field, first somewhat rapidly, then slowly, and then rapidly. The light is very bright. (d) The same procedure occurs as in (c), but now the light has been dimmed about 300 times. The vertical line shows the range of the photomultiplier, which has been adjusted for about 3½ decades of logarithmic response. (e) A 1-degree black disc is passed through the field at three speeds. (f) A 15-degree wide black strip is passed through the field at two speeds, edge leading. (g) A 15-degree wide black strip is passed through the field in various ways, corner leading. (h) The same strip as in (g) is passed through the field, edge leading.
Both groups I and II can detect targets as small as 3 minutes of arc in diameter and are affected by very faint blurring of the edge. Their resolution is thus quite high.
Myelinated fibers (Fig. 7)
These fibers form about 3 per cent of the optic-nerve population. There are three peaks of conduction velocity, one between 1 and 5 meters per second, one at 10 meters per second, and one at about 20 meters per second. Group III is responsible for the first peak, group IV for the second, and we think the efferents to the retina give the third.
Group III, the moving or changing contrast detectors (Fig. 7). These fibers have receptive fields, 7 to 11 degrees in diameter. They are, in effect, Hartline's “on-off” fibers with the differencing behavior
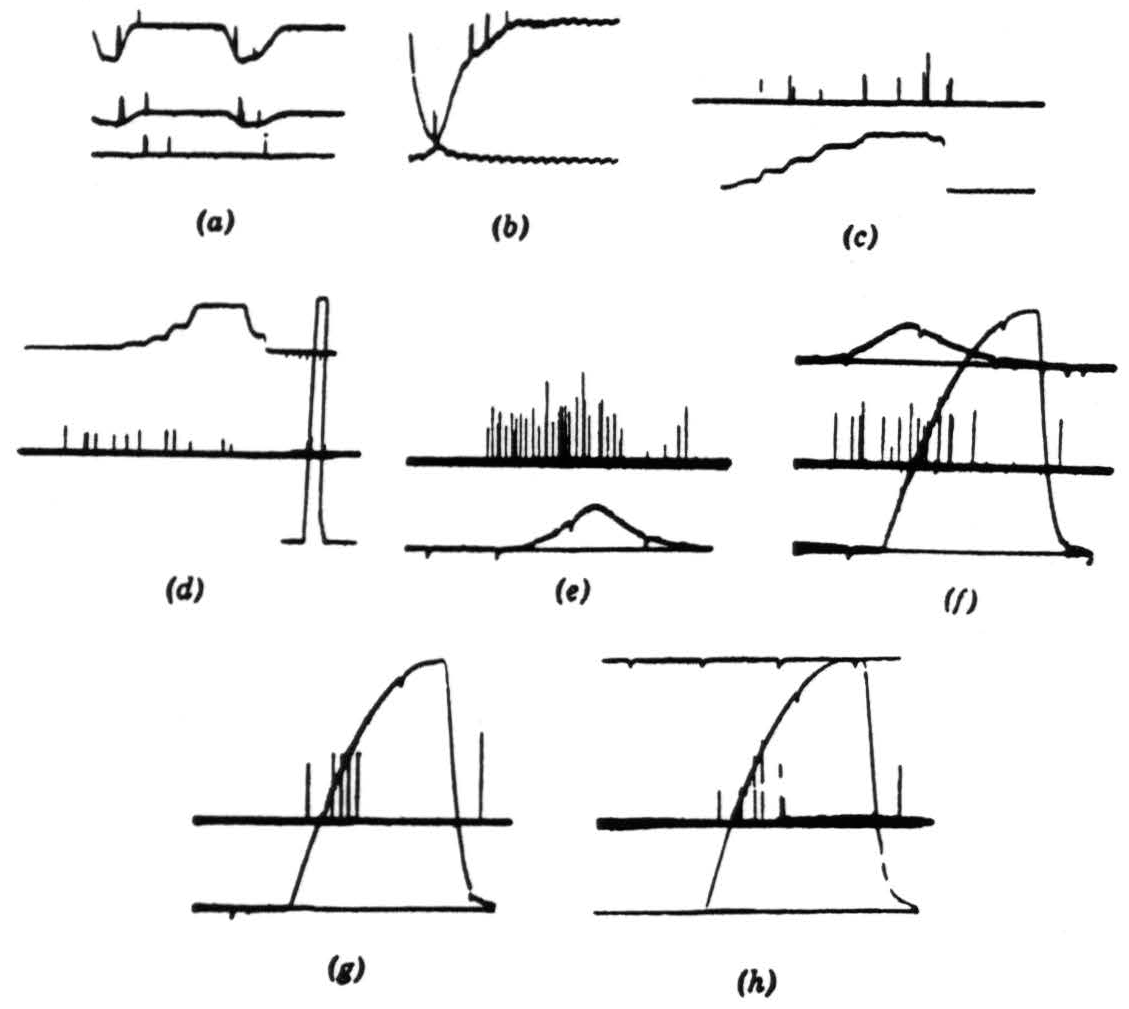
Figure 7. Operation 3. Moving-edge detectors. The first two pictures are taken from a single fiber in the optic nerve. (a) A 7-degree black disc moving through the receptive field (the photocell was not in registration with the field). There is a response to the front and back of the disc independent of illumination. There is about a 300-to-1 shift in illumination between top and bottom record. Darkening is a downward deflection with the photocell record. Time marks, 5 per second. (b) General lighting turned off and on. Time marks, 50 per second. Note double responses and spacing. (c) This and succeeding records are in the third layer of endings in the tectum. Several endings are recorded but not resolved. Darkening is an upward deflection of the photomultiplier record. The response is shown to the edge of a 15-degree square moved into and out of the field by jerks in bright light. (d) The same procedure occurs as in (c) but in dim light. Calibration figure is at the right. (e) The response is shown to a 7-degree black disc passed through the receptive fields under bright light. The sweep is faster, but the time marks are the same. (f) The same procedure as for (e) but under dim light. The off and on of the photomultiplier record were superimposed for calibration. (g) Off and on response with about half a second between on and off. (h) Same as (g) but with 2 sec between off and on.
reported by Barlow.(6) However, they respond invariantly under wide changes of illumination to the same silhouette moved at the
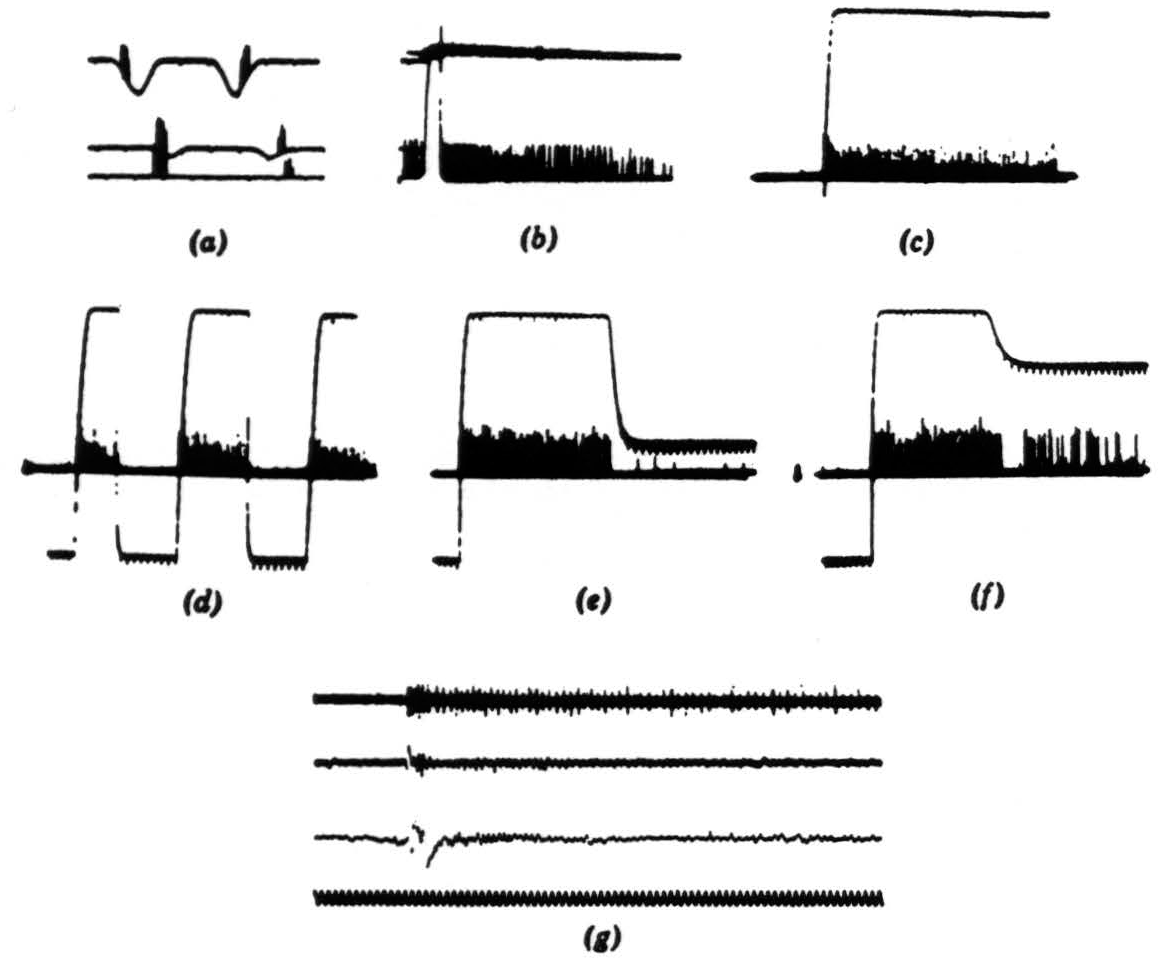
Figure 8. Operation 4. Dimming detectors. (a) This and the next frame are taken from a single fiber in the optic nerve. Here we see the response to a 7-degree black disc passing through the receptive field. The three records are taken at three illumination levels over a 300-to-1 range. In the phototube record, darkening is a downward deflection. Time marks, 5 per second. (b) Light turned off and on-off shortly after one sweep began, on a little earlier on the next sweep. The fiber is silenced completely by the on of the light. Time marks, 5 per second. (c) In this and the next three frames, we are recording from the fourth layer of endings in the tectum. This frame shows the response to turning off the general illumination. (d) Light off and on at regular intervals. (e) Light off and on at a lower brightness. (f) Light off and on at a still lower brightness. (g) The synchrony of the dimming detectors. At the top are three or four fibers recorded together in the optic nerve when the light is suddenly turned off. The fibers come from diverse areas on the retina. In the second line are the oscillations recorded from the freshly cut retinal stump of the optic nerve when the light is suddenly turned off. ln the third line are the oscillations recorded on the surface of the tectum, the visual brain, before the nerve was cut. Again the light is suddenly turned off. The last line is 20 cps. These records of synchrony were obviously not all made at the same time, so that comparing them in detail is not profitable.
same speed across the same background. They have no enduring response but fire only if the contrast is changing or moving. The response is better (higher in frequency) when the boundary is sharp or moving fast than when it is blurred or moving slow. Targets as small as 1 degree in diameter are effective throughout the whole receptive field. Sharp changes of illumination of a uniform field produce one or two on and off discharges. Local on or off in the center of the field produces more discharges over a longer period.
Group IV, the dimming detectors (Fig. 8). These detectors are Hartline's “off” fibers. They respond to any dimming in the whole receptive field weighted by distance from the center of that field. Boundaries play no role in the response. Thus resolution is poor. The same percentage of dimming produces the same response, more or less independent of the level of lighting at the beginning. Turning the light off produces an enduring response characterized by marked rhythmicity. The on intensity needed to interrupt the discharge completely and promptly decays with length of time after the off.
Unclassified
Group V is rare. We cannot even say whether it has a receptive field in the usual sense. It fires at a frequency that is inversely related to the intensity of the average illumination. When the lighting is changed, that frequency slowly changes to its new level. It may be significant that group V fibers end in the same layer of the tectal neuropile as do those of group III.
Argument
If we should assume that the size of the dendritic field to some extent determines the size of the receptive field, and that the size of the cell body is also related to the diameter of the axon, as seems to be the case elsewhere in the nervous system, we would emerge with a fairly definite correspondence between cell types and operations. The one-level constricted field would be associated with the boundary detectors, the one-level extended field with dimming detectors, the many-level H-shaped with the contrast movement and change detectors, the many-level E-shaped with the movement-gated, dark convex boundary detectors. The diffuse type, which is rare, we associate, more or less by default, with the average light-level measuring group. Our initial assumption depends for its validity mainly on the idea that the amacrine cells do not act in such a way as to invert the dendritic-field/receptive-field relation, and this seems to be the case for several reasons. First of all, the diameters of the dendritic fields match well the angular diameters of the receptive fields. Second, the cell bodies are distributed in size in the same way as the dendritic fields, and, if the axon diameters reflect soma size, then the largest axons ought to have the largest receptive fields and the smallest axons the smallest fields; and this seems to be the case. Finally, receptive fields often appear to be not circular but elliptical or cardioid. In Maturana's pictures of the ganglion cells, both elliptical and cardioid dendritic fields appear.
This identification of the physiological operation with the type of dendritic tree purely on the basis of dimensions leads us to further but very speculative deciphering. We have taken the inner plexiform layer as consisting of two major strata, not denying that further subdivision may be important. However, suppose that the output of a ganglion cell reflects the sort of information it collects. There are two types of one-level cell. The first, which is concerned with boundaries but not with changes in average intensity, arborizes at the inner stratum; and the second, which is concerned with changes in level of lighting but is indifferent to boundaries, arborizes at the outer stratum.
Can the two many-layered types be considered as different combinations of information about boundary and information about dimming? For example, let the H type take some function of dimming in all its receptive field and combine it with some function of boundary in all that field. Let the E type take some function, over all the small areas of its receptive field, of how much dimming there is in any one small area combined with how much boundary there is in that area. One would expect, from such a view, that the E type could resolve more complex geometrical properties of the image in its receptive field than could the H type. For the E type can be thought combinatorial of many small dendritic processes of the H type. Thus we consider the H type as an event detector, responding to any change in distribution of light and boundaries on its receptive field. The E type, however, is more constrained, in that a change in distribution of light must be coherent with change in sharp boundaries. Therefore, the H type matches with the changing contrast detectors and the E type with the dark convex boundary detectors, and this match is consistent with relative dimensions of receptive and dendritic fields.
A much more specific guess, however, concerns the nature of the bipolars and their relations to the photoreceptors. The explicitness of Ramon y Cajal almost ensures that the information coming to the outer levels of the inner plexiform layer is concerned mainly with the activity of the cones (those bipolars that connect only to cones and have broad dendritic spreads in the outer plexiform layer), whereas the information to the inner levels is combinatory of the different photoreceptors (those bipolars that connect to rods and cones and have very constricted dendritic fields in the outer plexiform layer). Let us suppose for the moment that there are but two sorts of receptor, the red rods and the cones. Suppose again that the receptors somehow or other have an effect on the bipolars that is logarithmically related to the amount of light incident—after all, this can be argued from the dynamic range of the retina and the Weber-Fechner law. If the effects of the rods and cones on the bipolars (connected to both sorts of receptors) are opposite, then those bipolars connected only to cones ought to measure some function of total illumination or its change, whereas those connected to rods and cones ought to measure some function of local differences—in a word, something about contrast and boundaries—and that measure should be independent of average illumination. That this may in fact be the case is suggested by the work of Svaetichin and McNichol(7) who, impaling an element somewhere between the outer and middle granular layers of the fish, found that as light intensity increased they got either continuous increases of depolarization or overpolarization of the element, depending on the color of the light.
It is very tempting to go further and construct a wonderfully complex model accounting for three photoreceptors, their relative densities, and so forth. But it would also be silly, for there are infinitely many that one may make (we have made some). The general notion that one sort of bipolar cell may “see” different kinds of photoreceptors as opposed in action, while another sort of bipolar sees one kind of photoreceptor only, is really the nucleus of the second guess and seems to be worth exploring. This notion is suggested by the anatomy; it is convenient to our deciphering of the ganglion cell function; it is not contradicted by any facts we know; and in fact it is supported to some extent by work on retinas of fish.
Part II. Collicular Cell Activity and Efferents to the Retina
In our other two papers we noted that the optic nerve fibers, having reached the colliculus, separate into four separate concentric sheets of terminals in the outer neuropile; that each stratum displays a continuous map of the retina in terms of one of the first four operations; that all four maps are in registration; and that, from the surface in, they occur in the order given, boundary detectors first, dimming detectors deepest. We also examined the cells in the tectum which receive from these terminals (Fig. 9). The great majority of them lie in the granular layer subjacent to the external neuropile and send their dendrites up to the very surface of the colliculus, as in Pedro Ramon's drawing. Some cells, but very few, lie in the external neuropile and look like horizontal connectives; these we shall not discuss. The cells in the granular layer do not emit their axons there; rather
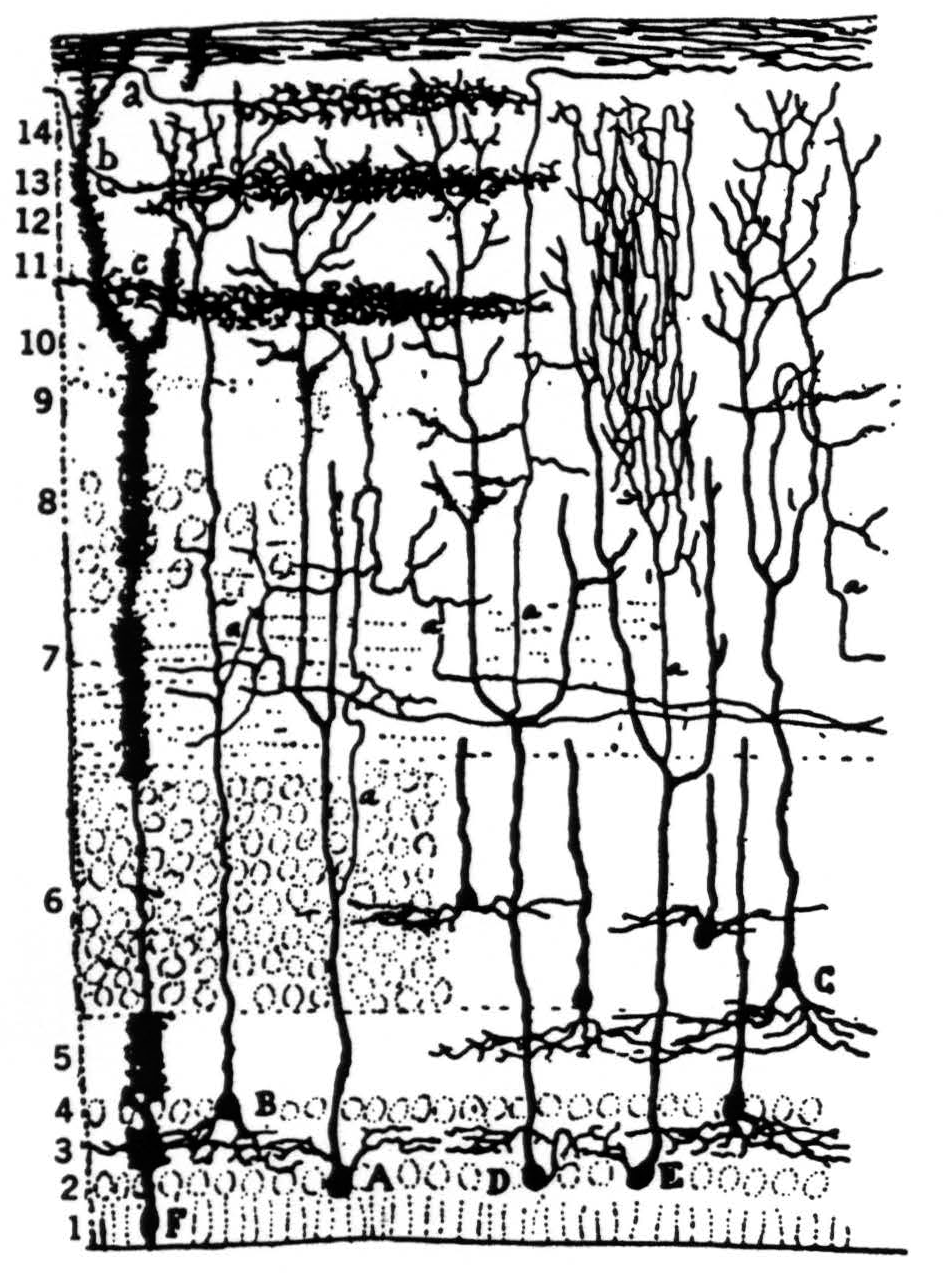
Figure 9. Pedro Ramon's drawing of optic tectum in the frog.
the axons arise from the large ascending dendrites and travel laterally in what we call the “palisade layer” which separates the dimming detector endings from the granular layer.
There seem to be several kinds of these cells, as one might expect. We have not been able to define the subgroups at all well, but there are two major populations of polar types which we have named “newness” neurons and “sameness” neurons. The former have axons which can be seen physiologically in the optic nerve, so they are part of the efferent system. Their cell bodies lie in the outer zone of the granular layer beneath the palisade layer. The somata of the “sameness” neurons lie deep in the granular layer, very close to the ependyma.
Our description of the operations is not definitive—we are far from satisfied. Nevertheless it gives the flavor of the responses we have seen.
“Newness” neurons
These cells have receptive fields about 30 degrees in diameter, twice that of the dimming detectors. They are distributed so as to map continuously the visual field with much overlap. Such a neuron responds a little to sharp changes in illumination. If an object moves across the receptive field, there is a response whose frequency depends on the jerkiness, velocity, and direction of the movement, as well as on the size of the object. There is never an enduring response.
Different cells have different optimum directions for movement and different preferred sizes of objects. But what is remarkable about the responses of such a unit is a kind of adaptation which is best described thus: if we move an object along any particular diameter of the receptive field in one direction and get a response, then repeating that movement over the same path in less than 5 to 10 sec brings no response. Even with a 20-sec delay to the second stimulus, the second response is much reduced below the first. But the adaptation is only to that path. If we move the object for the stimulus along another diameter, at a 90-degree angle to the first, then the first stimulus does not seriously interfere with the second. Both paths can be adapted within a second, whereupon a third stimulus running over any diameter will not be very effective in under 10 sec. In some of these units the adaptation can be erased by a moment of complete darkness. One variant we have seen is the response to centrifugal movement reported by Andrew(8) several years ago on our laboratory. A good fraction of such cells have transient responses to turning on or off the general illumination.
“Sameness” neurons
Each of these cells sees all or almost all of the visual field but has a “null region” that is different for different cells. We cannot say whether the null patches are so distributed as to map the retina in this layer. It is a bit embarrassing to present the following description so batrachomorphically, but at least it reflects what we have found so far. Every such cell, in fact, acts so complexly that we can hardly describe its response save in terms ordinarily reserved for animal behavior.
Let us begin with an empty gray hemisphere for the visual field. There is usually no response of the cell to turning on and off the illumination. It is silent. We bring in a small dark object, say 1 to 2 degrees in diameter, and at a certain point in its travel, almost anywhere in the field, the cell suddenly “notices” it. Thereafter, wherever that object is moved it is tracked by the cell. Every time it moves, with even the faintest jerk, there is a burst of impulses that dies down to a mutter that continues as long as the object is visible. If the object is kept moving, the bursts signal discontinuities in the movement, such as the turning of corners, reversals, and so forth, and these bursts occur against a continuous background mutter that tells us the object is visible to the cell.
When the target is removed, the discharge dies down. If the target is kept absolutely stationary for about two minutes, the mutter also disappears. Then one can sneak the target around a bit, slowly, and produce no response, until the cell “notices” it again and locks on. Thereafter, no small or slow movement remains unsignaled. There is also a place in the visual field, different for different cells, that is a sort of Coventry to which a target can retire and escape notice except for sharp movements. This Coventry, or null patch, is difficult to map. The memory that a cell has for a stationary target that has been brought to its attention by movement can be abolished by a transient darkness. These cells prefer small targets, that is, they respond best to targets of about 3 degrees.
There is also (we put this matter very hesitantly) an odd discrimination in these cells, which, though we would not be surprised to find it in the whole animal, is somewhat startling in single units so early behind the retina. Not all “sameness” cells have this property. Suppose we have two similar targets. We bring in target A and move it back and forth along a fixed path in a regular way. The cell sees it and responds, signaling the reversals of movement by bursts. Now we bring, in target B and move it about erratically. After a short while, we hear bursts from the cell signaling the corners, reversals, and other discontinuities in the travel of B. Now we stop B. The cell goes into its mutter, indicating that what it has been attending to has stopped. It does not signal the reversals of target A, which is moving back and forth very regularly all the time, until after a reasonable time, several seconds. It seems to attend one or the other, A or B; its output is not a simple combination of the responses to both.
These descriptions are provisional and may be too naturalistic in character. However, we have examined well over a hundred cells and suspect that what they do will not seem any simpler or less startling with further study. There are several types, of which the two mentioned are extremes. Of course if one were to perform the standard gestures, such as flashing a light at the eye, probably the cells could be classified and described more easily. However, it seems a shame for such sophisticated units to be handled that way—roughly the equivalent of classifying people's intelligence by the startle response.
Some axons of the “newness” neurons have been seen in the optic nerve. To a degree we suspect their action to be reflected (?) in the response of the movement-gated, dark convex boundary detectors—or perhaps it is the other way round. At any rate, in a convexity detector, if we move a small dark object in rapidly and stop, we can get a continuing response after the stop unless the object has been moved in along the same path immediately before, within, say, a few seconds.
All these comments apply only to the frog. That must be emphasized. Neither the anatomy nor the receptive-field operations are the same in mammals or even in other amphibia.
Summary
Part I. We propose that a deciphering of retinal anatomy is possible. We find a correspondence in number between operational groups at the output of the retina and anatomical groups. The relative size of dendritic field to receptive field is such that the anatomical and functional groups can be matched. There are significant differences in the shapes of dendritic fields of different size and there are equally great differences in the operations done by fibers with receptive fields of different sizes. In attempting to decipher the anatomical differences by means of the functional differences, we come to the hypothesis that (with respect to the discrimination of silhouettes) the inner levels of the inner plexiform layer are concerned with boundaries, whereas the outer levels are concerned with average (or changes in the average) illumination. This hypothesis can be extended to account for the fact that bipolar cells that end exclusively in the outer levels of the inner plexiform layer are connected only to cones, whereas those that end deepest in the inner layers are connected to both sorts of rods as well as the cones. Our supposition is that those bipolar cells that receive information only from cones report average, or changes in average, illumination, whereas those that receive from rods and cones report the two types of photoreceptors in opposition to each other. If the photoreceptors affect the bipolars logarithmically with respect to the exciting light, the latter sort of bipolar cell will report some function of boundaries invariant under change of illumination. The different operations seen at the output of the retina can be considered combinatory of the sort of information we suppose comes to the inner plexiform layer via the bipolar cells. The different combinations we think are got by different ways of distributing dendrites.
Part II. We discuss two sorts of cells in the colliculus that receive information from the optic nerve fibers. One of them is concerned with detection of novelty in visual events, and the other with continuity in time of interesting objects in the field of vision.
Footnotes
Acknowledgments
This work was supported in part by the U. S. Army (Signal Corps), the U. S. Air Force (Office of Scientific Research, Air Research and Development Command), and the U. S. Navy (Office of Naval Research), and in part by Bell Telephone Laboratories.
Dr. Maturana is on leave from the University of Chile, Santiago, Chile.
References
Lettvin, J. Y., H. R. Maturana, W. S. McCulloch, and W. H. Pitts. What the frog's eye tells the frog's brain. Proc. Inst. Radio Engr., 1959, 47, 1940-1951.
Maturana, H. R., J. Y. Lettvin, W. H. Pitts, and W. S. McCulloch. Physiology and anatomy of vision in the frog. J. gen. Physiol., 1960, 43 Suppl., 129-175.
Cajal, S. Ramon y. Die Retina der Wirbelthiere. Wiesbaden: J. F. Bergmann, 1894.
Maturana, H. R. Number of fibres in the optic nerve and the number of ganglion cells in the retina of Anurans. Nature, Lond., 1959, 183, 1406.
Hartline, H. K. The response of single optic nerve fibers of the vertebrate eye to illumination of the retina. Amer. J. Physiol., 1938, 121, 400-415.
Barlow, H. B. Summation and inhibition in the frog's retina. J. Physiol., 1953, 119, 69-88.
Svaetichin, G., and E. F. McNichol, Jr. Retinal mechanisms for chromatic and achromatic vision. Ann. N. Y. Acad. Sci., 1958, 74, 385—404.
Andrew, A. M. Report on frog colliculus. Quart. Prog. Rep., Res. Lab. Electronics, Mass. Inst. Technology, 1955, 77-78.
For further research:
Wordcloud: Axons, Bipolar, Black, Boundary, Cells, Change, Cones, Connected, Degree, Dendritic, Detectors, Diameter, Different, Dimming, Distribution, Fibers, Field, Fig, Figure, Frog, Ganglion, Group, Illumination, Inner, Layer, Levels, Light, Moved, Movement, Nerve, Object, Operations, Optic, Outer, Per, Plexiform, Receptive, Records, Respond, Response, Retina, Rods, Several, Sharp, Size, Small, Sorts, Target, Turned, Type
Keywords: Nerve, Cells, Frog, Image, Shows, Fibers, Relations, Arbors, Systems, Cognition
Google Books: http://asclinks.live/xrgp
Google Scholar: http://asclinks.live/pyg3
Jstor: http://asclinks.live/y75x