FUNCTIONAL ORGANIZATION THE SENSORY CORTEX OF THE MONKEY (MACACA MULATTA)1 [20]
J.G. Dusser de Barenne and W.S. McCulloch
Introduction
In 1924(1, 2) the location and extent of the sensory cortex were established in the brain of the monkey (Macaca) by means of the method of local strychninization. This cortex was found to occupy a large portion of the post- and precentral region, and to comprise three major subdivisions (the leg-, arm- and face-subdivisions). This mapping was achieved by the local application of minute quantities of strychnine to very small portions of the cortex; such applications, if performed within the sensory cortex, resulting in marked symptoms of sensory excitation (paraesthesiae, hyperaesthesia and hyperalgesia) in different parts of the body.
Such an application of strychnine not only produces symptoms of hypersensitivity but also typical changes in the electrocorticogram (ECG),2 namely the appearance of large, rapid potential fluctuations—“strychnine-spikes.” The occurrence of such spikes is not limited to the sensory cortex; they appear upon local strychninization of any portion of the cerebral cortex. The distribution of the spikes, however, is dissimilar when the areas are dissimilar.
It is first of all with these dissimilarities in distribution that the present paper deals, both in regard to the distribution of the spikes within the cytoarchitectonic area, of which a part has been strychninized, and in regard to the distribution of the spikes in architectonic areas other than the area locally strychninized. Furthermore this paper deals with the observation that strychninization of two definite areas results, besides producing strychnine-spikes in other areas, in a temporary suppression of the electrical activity of another area. Finally the results obtained with strychninization were corroborated in experiments with electrical stimulation of various areas and recording of the electrical after-discharge in other areas.
Methods
The experiments were all performed on monkeys (Macaca mulatta), either fully anesthetized with Dial3 (0.45 cc. per kilogram, half of the dose given intraperitoneally, half intramuscularly), or ether, or operated upon under Vinylether4 and curarized, but studied while awake. The strychnine (3 per cent solution, colored with toluidine blue) was always applied to a very small portion of the cortex, measuring only 1-4 square mm., either by touching the cortex at the desired locus with a pledget of cotton wool twisted around the ends of an irisforceps and moistened with the strychnine solution or by covering the cortex with a very small piece of filter paper, soaked with the strychnine solution. Before application of the pledget or filter paper the excess strychnine solution was carefully removed from it. For brevity we will refer to the application of strychnine to a few square millimeters of cortex, by either procedure, as “local strychninization.”
The electrocorticograms were taken from various portions of the cortex with two fine Ag-AgCl electrodes, 3 to 4 mm. apart, this being the optimal distance for recording the ECG with this type of electrodes. In most of the experiments the electrical activity was recorded with a cathode ray oscillograph after suitable amplification through a two-stage DC-amplifier. This method permits only successive electrocorticograms. Later AC-amplification with a four-element Westinghouse oscillograph was used for simultaneous recording of four electrocorticograms. The results of these experiments confirmed the previous observations. In many experiments bipolar electrical stimulation of one cortical focus was performed and the changes in the electrocorticograms (electrical after-discharge) of various foci examined.
Results
In Figure 1 is shown the location and extent of the sensory cortex of the brain of Macaca mulatta, with the subdivision into its leg- arm-, and face-areas.(1)
It should be pointed out that, apart from the incorporation of the cytoarchitectonic denominations, the present diagram differs in two respects from that of 1924, namely in the relation of the beginning and end of the sulcus interparietalis to the various subdivisions. The diagram of 1924 was based upon the study of 20 monkeys, in which only a few experiments could be devoted to the areas in question. The present diagram is based upon a large number of experiments and represents more truly an “average” of the findings in relation to the configuration of the surface of the macaca’s brain, which differs so much from animal to animal.
Within each subdivision are also represented some of its cytoarchitectonic areas, simplified and modified after the investigations of Brodmann(3, 4) and C. and O. Vogt.(5) The main deviation from their diagrams is the inclusion in Figure 1 of a field 4-s between 4 and 6a. From our diagram it can be readily understood what is meant in this paper by local strychninization within arm 4 area (A.4), leg 6a area (L.6a), or face 2 area (F.2), etc.
1. Changes of the ECG in general following local strychninization.
Local strychninization of any portion of the cerebral cortex elicits typical changes in the ECG at the site of strychninization, namely the appearance of “strychnine-spikes.” The first alteration, within a few seconds after the application of the strychnine, is an augmentation of the general electrical activity of the cortex; 30 to 60 secs. later the strychnine-spikes begin to appear which are fully developed in about 2 to 3 minutes. With the development of the spikes the “background”-activity, i.e. the potential fluctuations between the spikes, often markedly diminishes. At the end of about 15 minutes, the spikes begin to decrease slowly in size and frequency, while the background-activity is returning to its original magnitude. In 20 to 40 minutes the ECG has usually regained its original characteristics. Renewed local application of strychnine then reinduces the whole train of events.
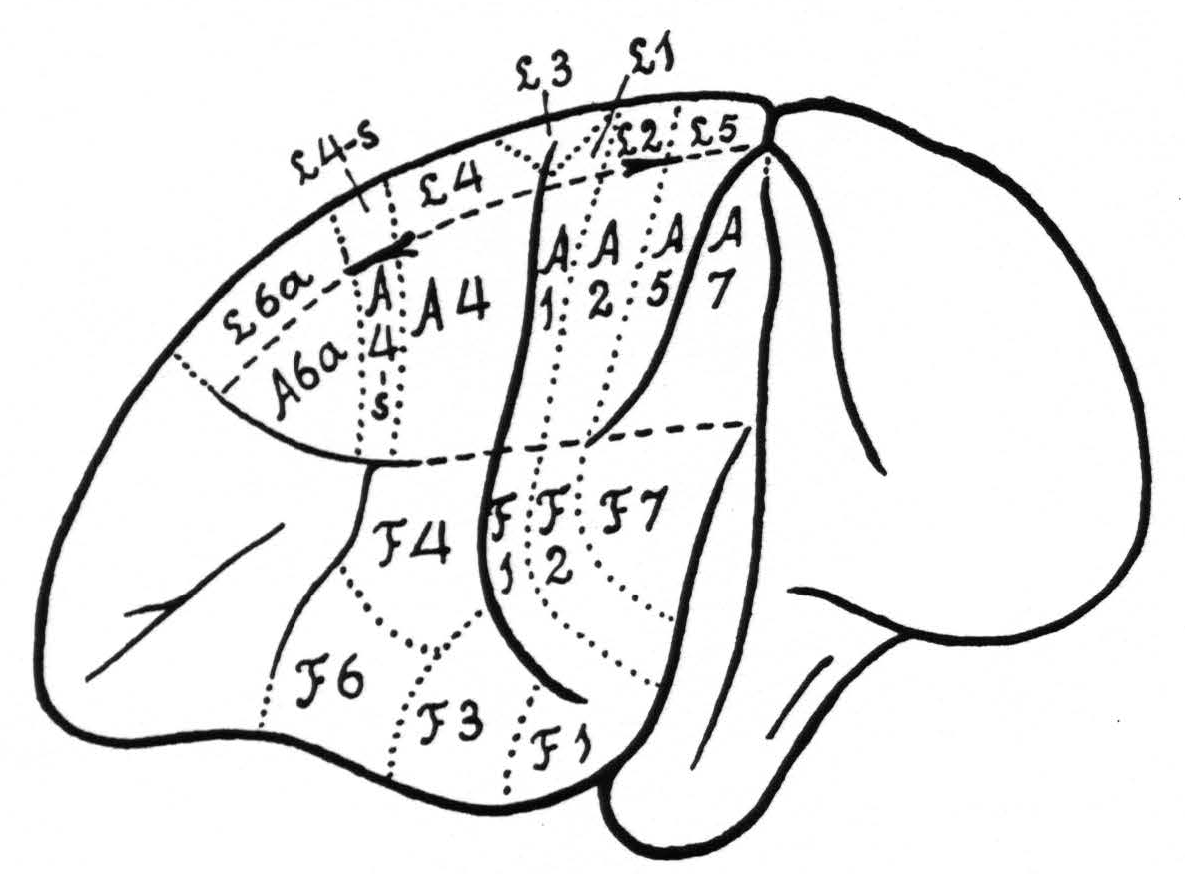
Figure 1. Extent and location of the sensory cortex in the brain of Macaca mulatta, with some of the architectonic areas, appearing on the surface of the brain (slightly modified after Brodmann and C. and 0. Vogt). The essential deviation from their diagrams is the introduction of an area L.4-s and A.4-s.
The configuration of the strychnine-spikes, recorded from the surface of the hemisphere, depends upon a number of factors, of which the most important are the size and positions of the electrodes and the stage of strychninization. We cannot enter here into a discussion of these relations; they have been dealt with to some extent at the Atlantic City meeting of the American Neurological Association, June, 1937 (See Transactions of that meeting).
Local strychninization anywhere within the sensory cortex (see Figure 1) produces spikes not only in the ECG of the minute area strychninized, but also in other portions of the sensory cortex. The distribution of these spikes is wide-spread, but not fortuitous. It is constant for any given area, but different for the various cytoarchitectonic areas and the various major subdivisions of the sensory cortex. This distribution is altered neither by deep undercutting of the whole sensory cortex, nor by thermocoagulation of a narrow circular strip of cortex, throughout its entire thickness, around the area strychninized.
2. Distribution of the strychnine-spikes within the area locally strychninized.
The distribution of the spikes within an area locally strychninized differs for various regions of the cerebral cortex. This distribution is very restricted within the visual cortex (area 17 of Brodmann) and within area 5 of the sensory cortex: large spikes are present at the site of strychninization, small spikes in its immediate vicinity. One or two millimeters and farther away from the strychninized locus spikes are absent (see Figure 2).
In other portions of the cerebral cortex the distribution of the strychnine-spikes is much wider. Local strychninization of A.4 for instance results in a “firing” of the whole of this area, i.e., results in the appearance of spikes in the ECG of all of A.4 (see Figure 3).
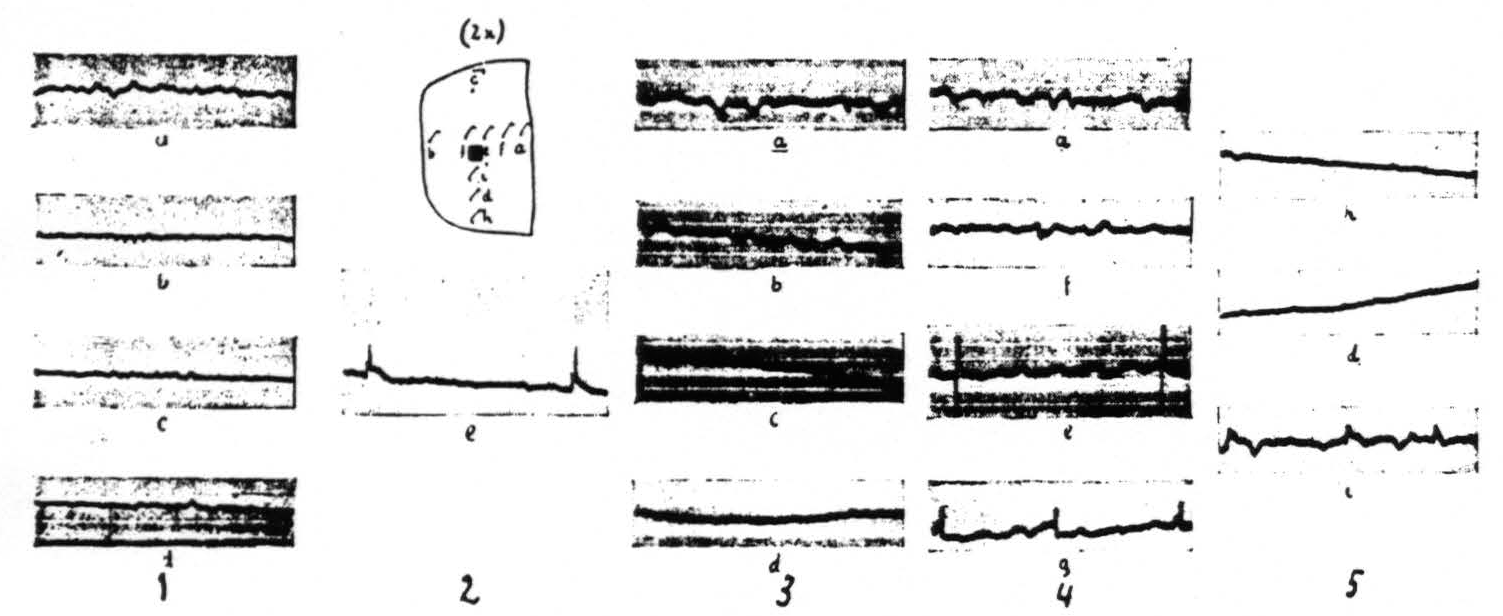
Figure 2 gives the results of local strychninization of the visual cortex (area 17 of Brodmann) and shows that the strychnine-spikes appear only in the immediate vicinity of the strychninized area. First column shows the ECGs before the strychninization. The other ECGs of the loci indicated by the letterings at different times after the strychninization. Same amplification throughout. Column 1 before, the other columns after the strychninization. ECG of column 2 (e) taken 2 minutes after, those of column 3, 4 minutes; column 4, 7 minutes and column 5, 10 minutes after the strychninization.
3. Distribution of the strychnine-spikes in other areas than the one strychninized.
One finds the widest distribution of the strychnine-spikes in the sensory cortex. The local strychninization of any cytoarchitectonic area within this cortex produces spikes in several other constituent areas. It is advisable to take up the various subdivisions of the sensory cortex separately. We shall first describe the findings relating to the arm-subdivision. Here the following results obtain: local strychninization of area 7 “fires” not only this area itself, but also areas 5, 2, 1 and 4; that of area 5 “fires” areas 2, 1 and 4; that of area 2 “fires” also areas 7, 5 and 4; that of area 1 “fires” also areas 7, 5 and 2; that of area 4 “fires” also areas 7, 5, 2 and 1.
In the case of the leg-subdivision the same results are found so far as the homologous areas, mentioned for the arm-subdivision, are concerned. In the leg-subdivision a portion of area 3 appears on the surface of the hemisphere, so that there this area can be locally strychninized on its surface. Such strychninization results in a “firing” of areas L.5, L.2, L.1, L.3 itself, and L.4. In the face-subdivision it was found that local strychninization of its post-central region “fires” this region and also the precentral portion, and vice-versa. As yet we have not enough experimental data to make a more detailed statement for this subdivision.
What is common to all of these cases is: (1) that strychnine-spikes are always largest in the area strychninized locally, and diminish in size with increase of distance of the area “set on fire” from the area strychninized; (2) that the distribution of the spikes is limited to the subdivision locally strychninized; and finally (3) that area 6a is not “fired."
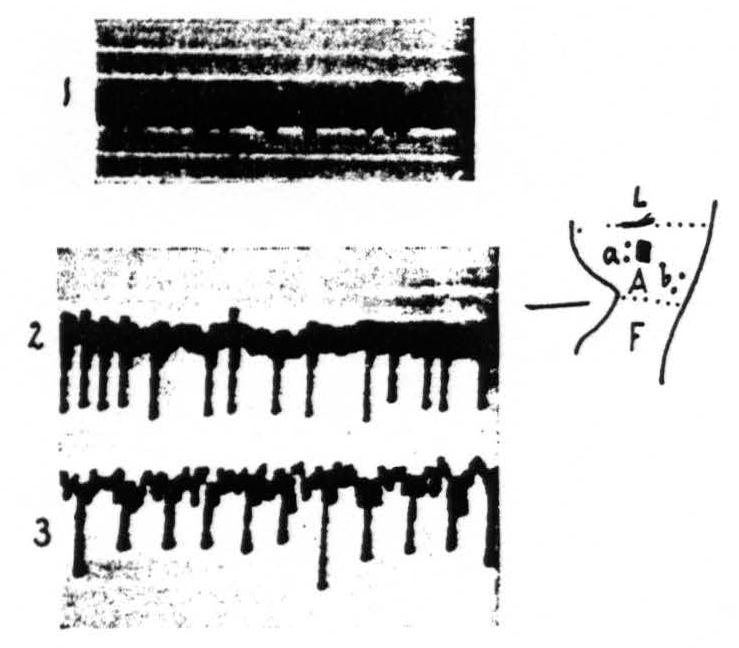
Figure 3 shows that local strychninization of A.4 is followed by large spikes throughout this area. Record 1 is ECG before strychnine-application. Record 2 gives the ECG at a, record 3 the ECG at b at the acme of the strychnine-spikes.
Figure 4 shows: (1) that local strychninization of A.5 does not “fire” this area, even in the vicinity of the locus strychninized; (2) that it “fires” A.4; (3) that it does not “fire” the precentral and postcentral region of the face-and leg-subdivisions. Figure 5 shows: (1) that local strychninization of L.4 “fires” L.4, L.2 and L.5; (2) that the spikes diminish in size the farther away the area “fired” is from L.4; (3) that the arm-subdivision is not “fired” and (4) that L.6a is not “fired."
In both Figures 4 and 5 the gradual return toward the normal ECG, present before the local strychninization, can be observed.
The sensory cortex situated in front of L.4 and A.4 requires separate mention. Here two areas, designated 6a and 4-s in Figure 1, can be differentiated.
First of all it should be stated that area 6a is the only portion of the sensory cortex which is not fired by the local strychninization of any other portion of the sensory cortex. Secondly, the local strychninization of either portion of
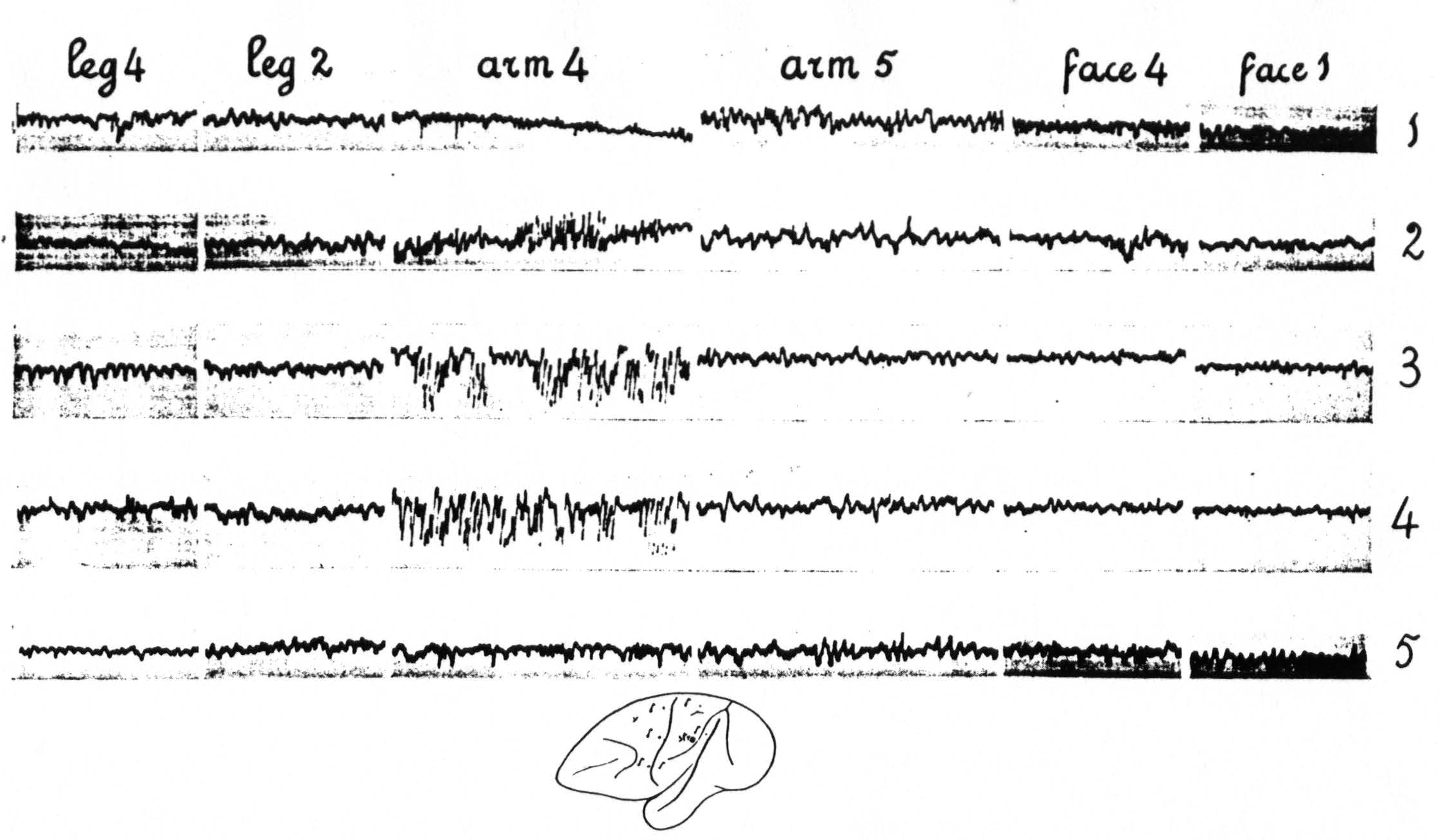
Figure 4 (experiment of April 10, 1936; Dial-narcosis) shows the results of local strychninization of A.5. No apparent changes in the ECG of A.5 immediately outside of the strychninized area. Enormous increase of the electrical activity in A.4. No changes in the ECG of L.2, L.4, F.1 (and F.2) and F.4 (functional boundaries between the three major subdivisions). Row 1 gives the ECGs before the local strychninization of A.5, rows 2, 3, 4 and 5 at different times after this strychninization. Note the return to normal of the ECG of A.4 in row 5.
area 6a, of L.6a or of A.6a “fires” all constituent areas of the leg- and of the arm-subdivisions, i.e. strychnine-spikes appear in L.6a, L.4, L.3, L.1, L.2, L.5, A.6a, A.4, A.1, A.2, A.5 and A.7.
The local strychninization of area 4-s produced equally striking results. Strychnine-spikes appeared in all of area 4-s, irrespective of the locus of strychninization in this area, i.e. in either A.4-s or L.4-s, and in the post-central portions of the arm- and of the leg-subdivisions. The new observation in this set of experiments was that a temporary suppression of the electrical activity of area 4 ensued, both in L.4 and in A.4. See Figure 6. Within a few minutes after the application of the strychnine to either L.4-s or A.4-s the electrical activity of L.4 and A.4 diminished considerably, while strychnine-spikes were present in the electrocorticograms of all the postcentral areas of the leg- and of the arm-subdivisions. After about 15 minutes, when the strychnine-spikes in these areas began to diminish in size and frequency, the ECG in area 4-s began to come back and 20 to 25 minutes after the application had returned to its normal magnitude, simultaneously with the disappearance of the strychnine-spikes in the other portions of the leg- and arm-subdivisions. No changes were apparent in the ECG of area 6a. This result is shown in Figure 6.
Areas 6a and 4-s have, therefore, this in common that no functional boundary between the arm- and leg-subdivisions is apparent so far as the changes in the ECG following local strychninization of these areas is concerned, both in regard to the distribution of the strychnine-spikes and in the case of area 4-s to the temporary suppression of the electrical activity of area 4.
The local strychninization of area 1, like that of area 4-s, suppresses temporarily the electrical activity of area 4, while “firing” the other postcentral areas of the sensory cortex; it does not produce any changes in the ECG of area 6a. In the case of the local strychninization of area 1, however, these changes in the ECG are restricted to the subdivision to which the minute strychninized portion of area 1 belongs. The results of the local strychninization of area 1 are shown in Figure 7.
4. Electrical stimulation of one locus producing electrical after-discharge in other loci of the sensory cortex.
From previous work(6) we knew that electrical stimulation of such a type (sufficiently long duration, long pulses, etc.) that when applied to the “motor” cortex motor after-discharge occurs, will at lower voltages still produce an electrical after-discharge, evidenced in the ECG, though no peripheral motor response is manifest. We have found that when any focus of the sensory cortex is thus stimulated the electrical after-discharge is present not only at the site of stimulation and its immediate vicinity, but also at other foci, in other areas of this cortex. Thus we have here a method to check the results obtained in the strychnine experiments.
It was found that the distribution of this after-discharge upon appropriate results in strychnine-spikes in ECG of L.4, L.2, L.5. No apparent change in ECG of Time interval between taking of the first ECG of each row ca 5 minutes. Note also the electrical stimulation of any one locus of the sensory cortex was essentially the same as the distribution of the strychnine-spikes following local strychninization of this same locus. For this reason it is unnecessary to give the results of these experiments in detail.
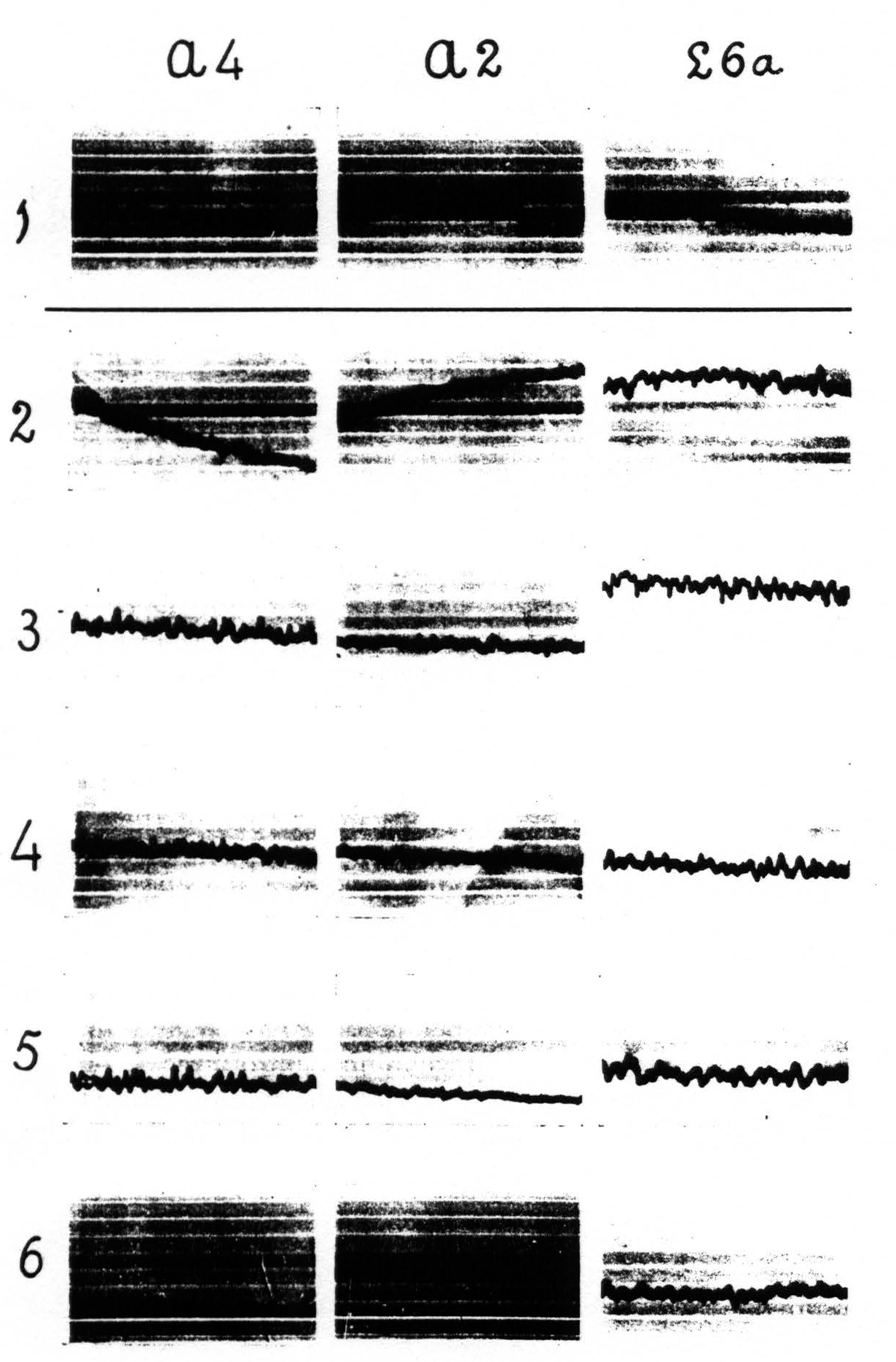
Figure 5. Experiment of April 23. 1936. Dial-narcosis. Local strychninization of L.4 L.6a, nor in ECGs of A.4 and A.2. Row 1 before, the other rows after the strychninization. gradual return to “normal” from row 4 on.
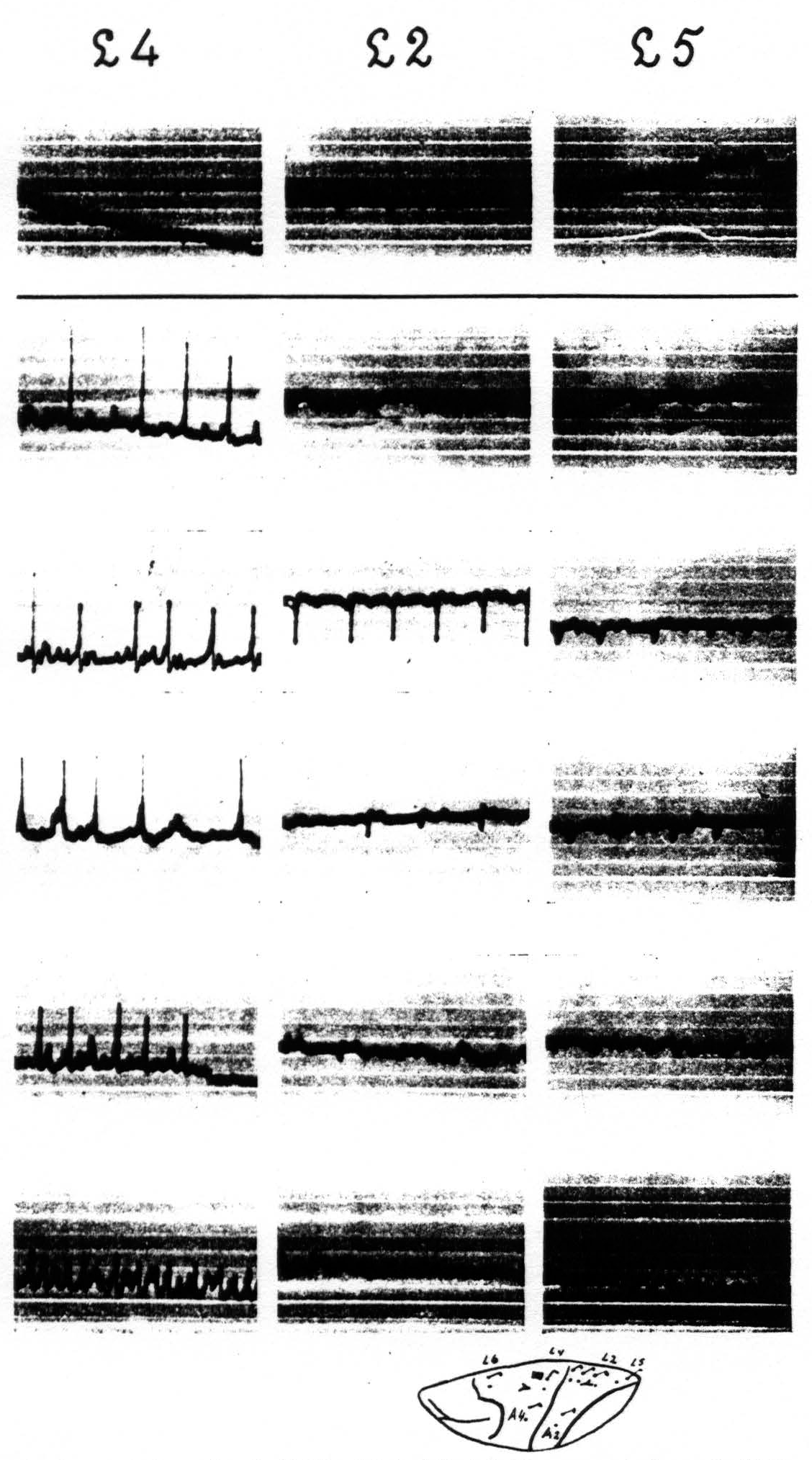
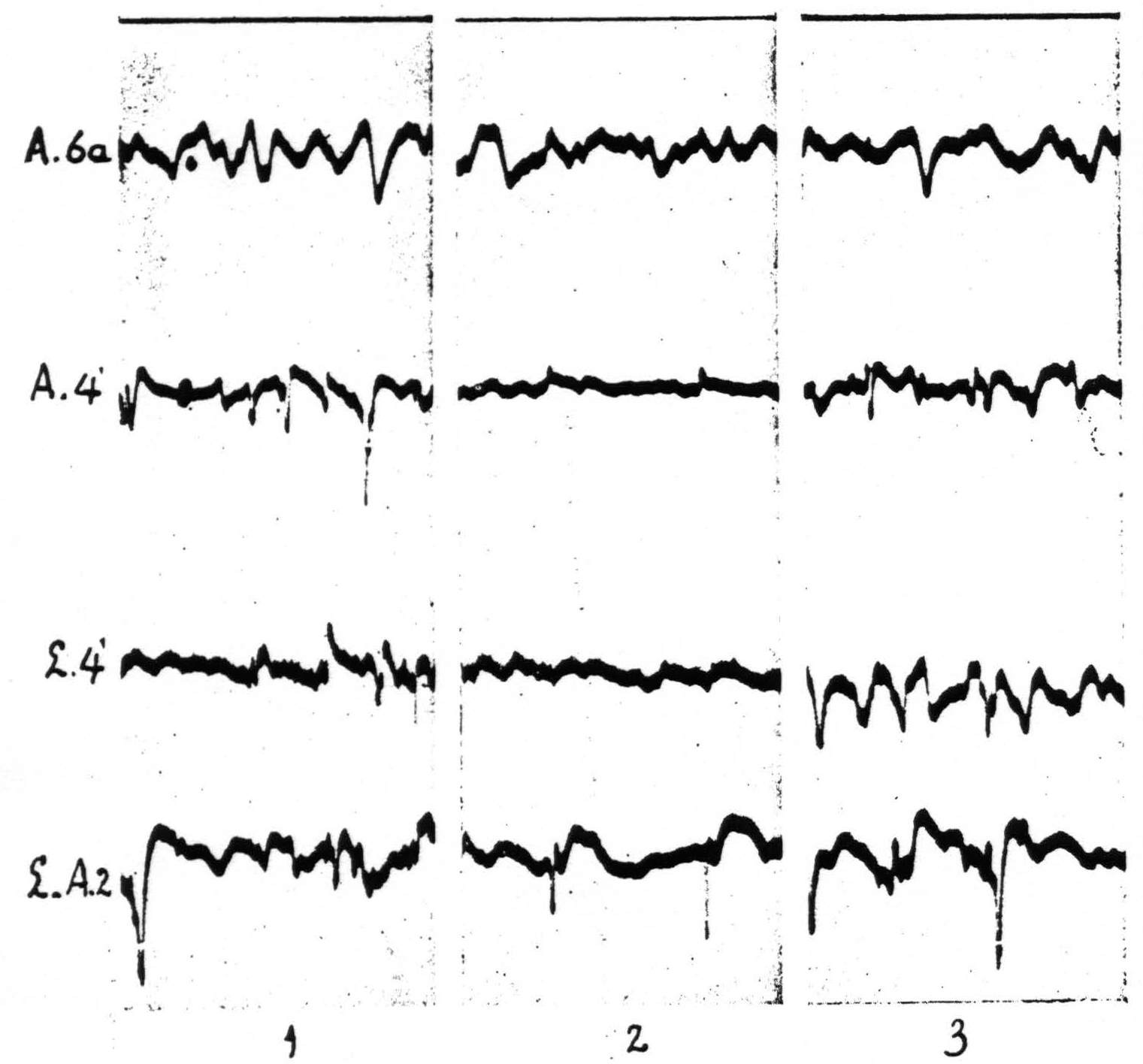
Figure 6 shows in column 2 the temporary suppression of the ECG of A.4 and L.4 upon local strychninization of A.4-s. Spikes in postcentral cortex (L-A.2 means that one of the two electrodes was on L.2, the other on A.2). No apparent change in ECG of A.6a. Records of column 1 before, those of column 2, 7 minutes, and those of column 3, 11½ minutes after the strychninization of A.4-s. Simultaneous ECGs with 4-element Westinghouse oscillograph.
In Figure 8, which shows the distribution of electrical after-discharge in the leg-subdivision of the sensory cortex following appropriate electrical stimulation of a focus of L.4 (4.5 Volt, 5″, 60∼) it will be seen that electrical stimulation of a focus of L.4 produces electrical after-discharge in L.4, L.2, and L.5, but not in L.6a. Comparison with Figure 5 shows the similarity of the distribution of the electrical after-discharge and the strychnine-spikes in these two sets of experiments, obtained by electrical stimulation and local strychninization of one and the same focus, with recording of the ECG from the same loci in the same cortex of the same animal.
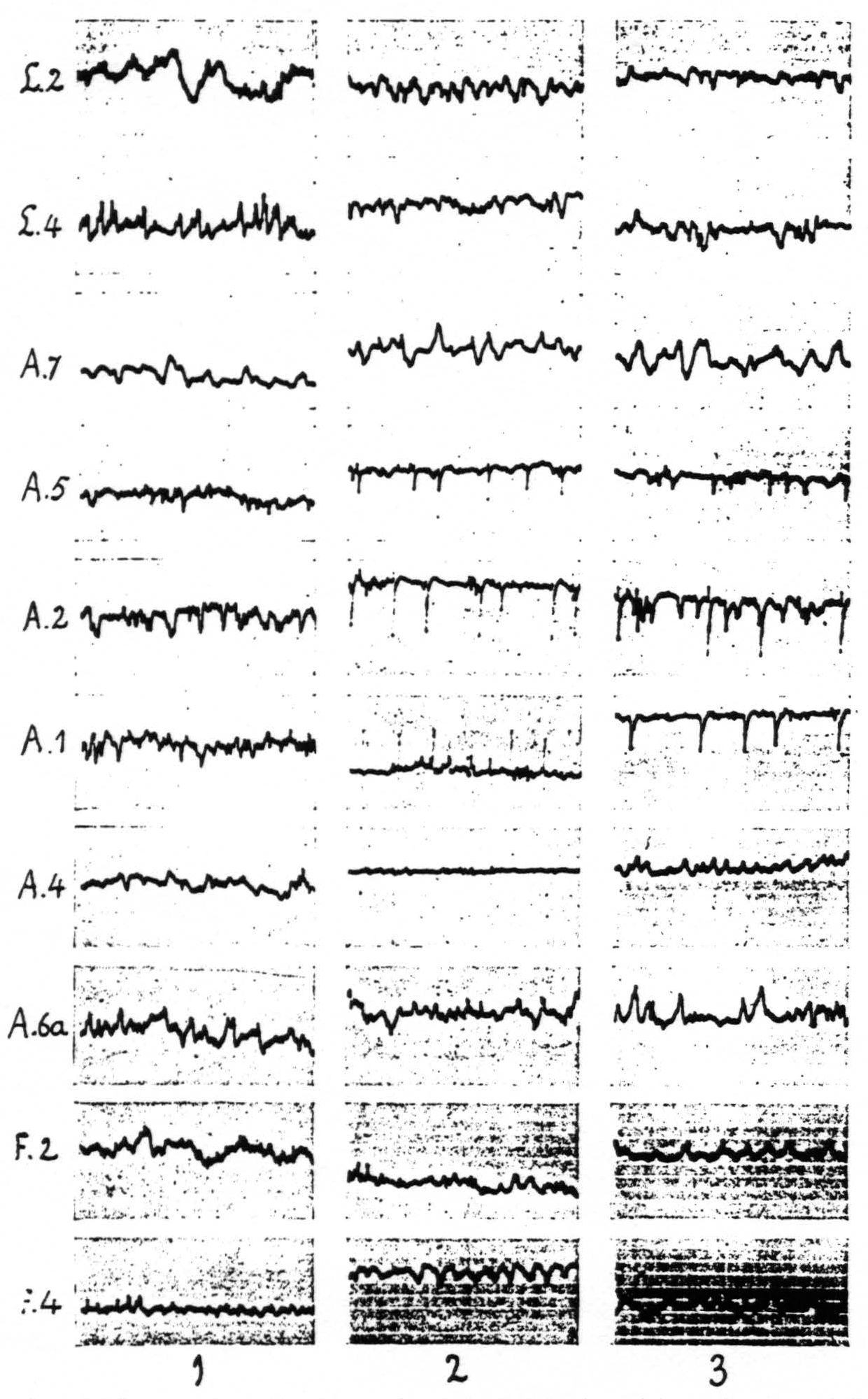
Figure 7. Figure 7 shows the effect of local strychninization of A.1. Spikes in A.1, A.2, A.5. Temporary suppression of ECG of A.4 in column 2. No changes in A.6a, nor in L.2, L.4, F.2 and F.4. Return to normal of ECG of A.4 in column 3. Records of column 1 before, those of columns 2 and 3 after the strychninization.
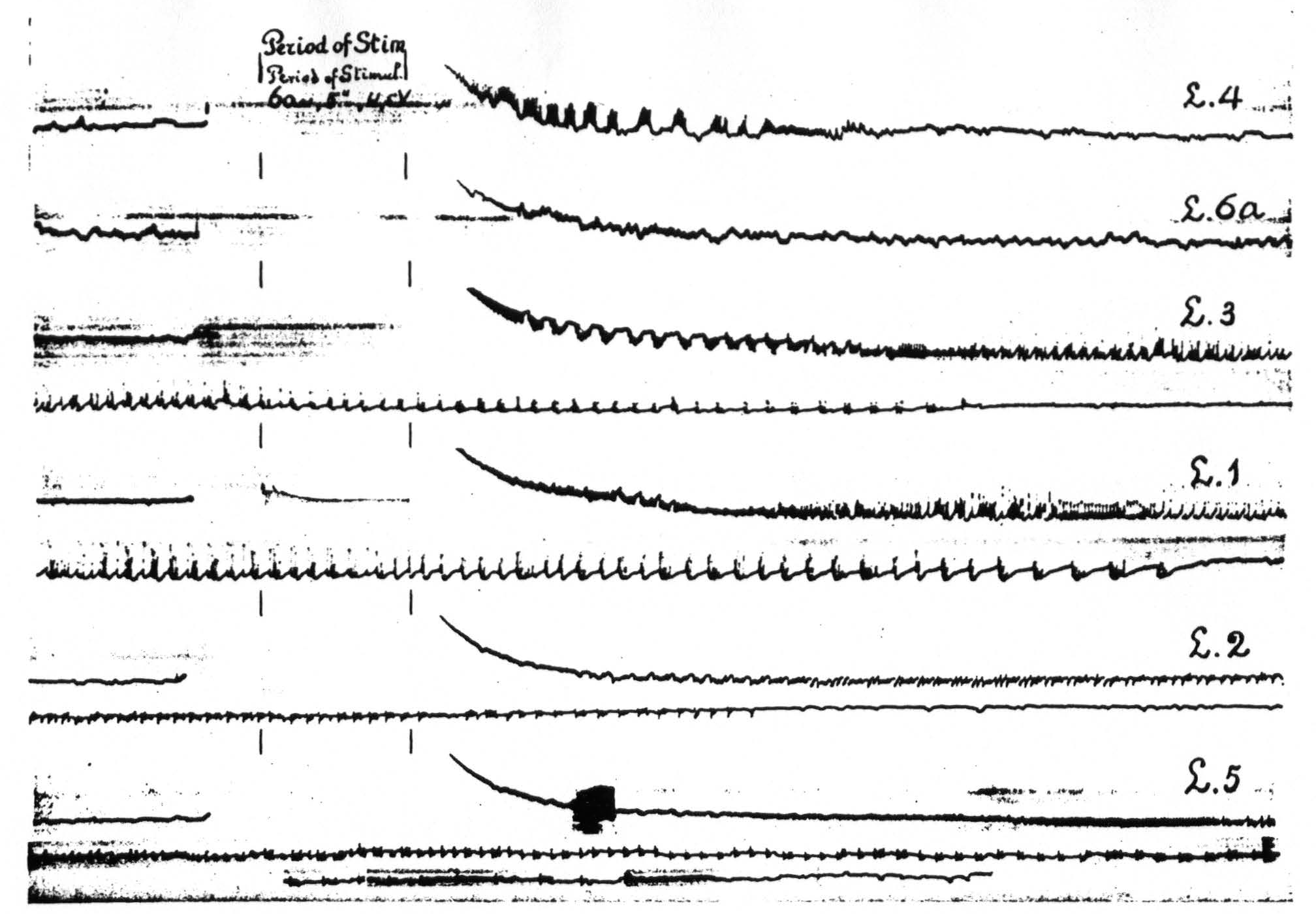
Figure 8. Experiment of April 23, 1936 on same animal and same foci as of Figure 5. All records taken before and after electrical stimulation (60∼, 4.5 V, 4 seconds) of a focus of L.4. Record 1 is ECG of L.4 itself, 2 that of L.6a, 3 of L.3, 4 of L.1, 5 of L.2, 6 of L.5. Note electrical after-discharge in all records, except in that of L.6a.
Discussion
The expression that local strychninization of one area “fires,” or “sets on fire,” this or another area is used purely for convenience to indicate the appearance of strychnine-spikes in this or that area. In 1912 one of us(7) adduced evidence indicating that strychnine produces its remarkable symptoms of sensory excitation, if applied locally to the dorsal horn of the spinal cord, by acting on the nerve cell bodies—the perikarya—within the strychninized area. It is plausible that this holds also for the cerebral cortex, both in regard to the symptoms of sensory excitation and to the typical changes in the ECG following local strychninization of the (sensory) cortex.
The observation that injection of strychnine into the white matter underneath the cortex—the corona radiata—does not produce sensory symptoms nor strychnine-spikes in the cortex (or the thalamus) is experimental evidence supporting the view that the strychnine produces its remarkable symptoms by action on nerve cell bodies. The fact that essentially similar spikes occur at the site of strychninization irrespective of the cytoarchitectonic structure of the area in question shows that the local functional change induced by the strychnine is uniform throughout the cortex and that the spikes cannot be attributed to the strychninization of any single type of nerve cell. The specific distribution of the spikes encountered in these experiments for any given area strychninized precludes explanation of the findings in terms of any diffusion or absorption of the strychnine. This is in agreement with the older observations, previously adduced by one of us, which all indicate the remarkably local action of strychnine, when applied locally.
When strychnine-spikes appear in various areas upon strychninization within one area these distant spikes are practically simultaneous with the spikes in the area strychninized. Recording on fast-moving film or paper, however, shows that there is a delay of a few sigmas, the longer the greater the distance between the area strychninized and the area from which the spikes are recorded. Whether these propagated, distant spikes are merely axonal action-potentials or whether they reflect also the activity of nerve cells excited by these axons, is not settled. The long duration of the last phase of most of the spikes would seem to indicate that they can not be regarded entirely as pure axonal potentials.
Whatever the explanation of the strychnine-spikes may be, the fact that an area a “fires” an area b, whereas b does not “fire” a, must be the expression of directed functional relations, and, therefore, directed anatomical relations, between these two areas. Assuming that strychnine produces the spikes by acting on the perikarya of the cortex, the finding mentioned above must be interpreted to mean that nerve cell bodies in area a send their axons into area b, whereas b has no cell bodies the axons of which extend into a.
It is possible to make a few general statements about the course of these interareal axons. The distribution of the spikes is not changed by the thermo-coagulation of a narrow circular strip of cortex over the entire thickness of the cortex around the strychninized locus nor by deep undercutting of the whole sensori-motor cortex. These findings prove that axons from the area strychninized run through the white matter of the pallium to those other areas in which strychnine-spikes are present. Even within one area, e.g. A.4, circumthermocoagulation of the locus strychninized does not prevent spikes from appearing throughout that area, so that intraareal as well as interareal axons pass through the subjacent white matter. Obviously, these findings do not preclude the operation of intracortical, i.e. intragriseal, axons, but they do show ,that such axons are not necessary for the apparently normal distribution
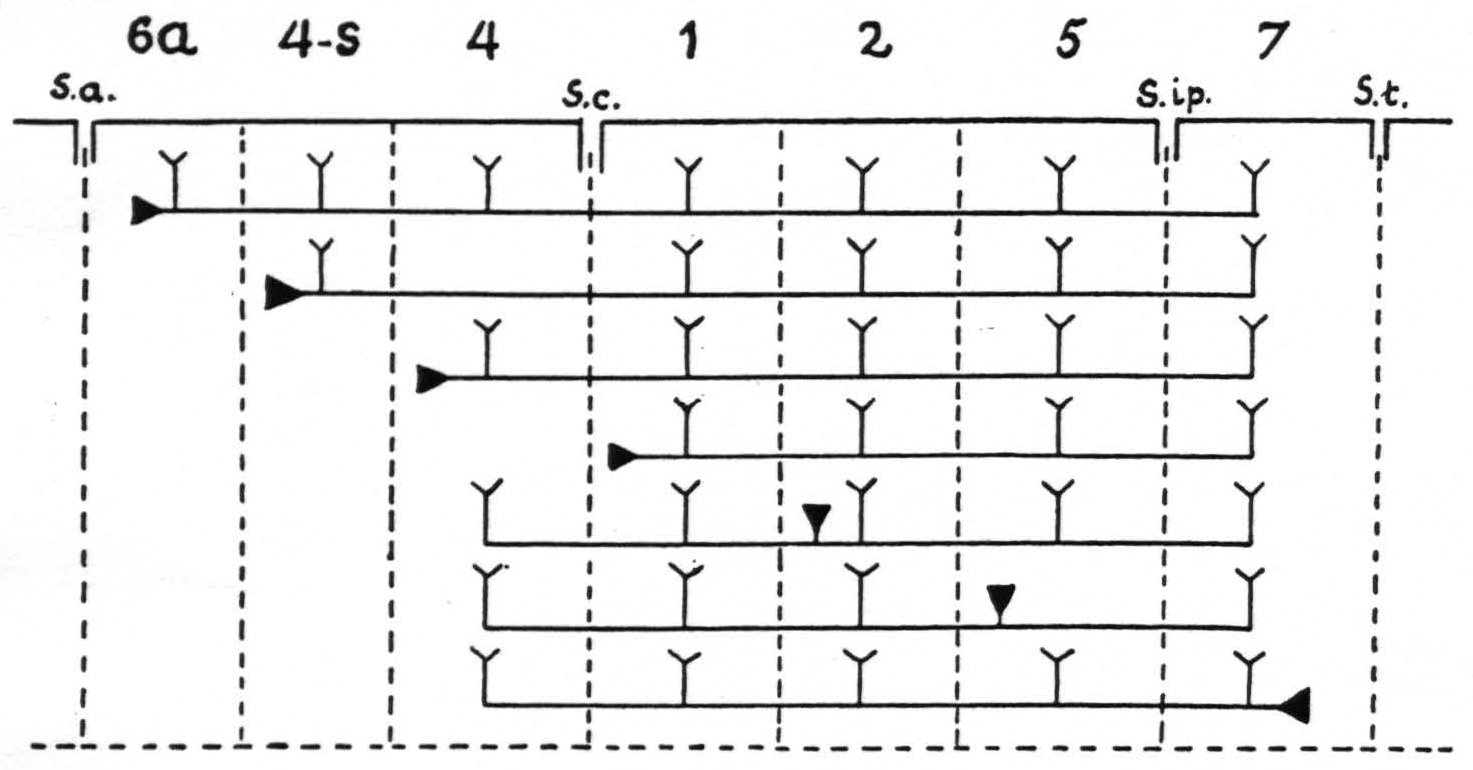
Figure 9. In Figure 9 are represented diagrammatically the directed functional (and anatomical) relations between the various cortical areas of the arm-subdivision of the sensory cortex found in these experiments. The suppression of the ECG of area 4 upon local strychninization of area 4-s or area 1 is not represented, because this is not dependent upon cortico-cortical relations. s.a. =sulcus arcuatus; s.c. =sulcus centralis; s.ip. =sulcus interparietalis; s.t. =sulcus temporalis I.
of the strychnine spikes. The specific intraareal and interareal, directed functional relations within the sensory cortex, schematised on the basis of the view that strychnine produces spikes by action on the perikaryon, are given diagrammatically in Figure 9. For reasons of simplicity, the axons are drawn as remaining inside the “griseum” of the cortex, although, as stated in the preceding paragraph, they actually run through the subjacent white matter.
The feature common in the phenomena observable after local strychninization of 6a or 4-s is the appearance of strychnine-spikes in both leg- and arm-subdivisions of the sensory cortex, although the strychninization was performed in only one of the following areas: either L.6a or A.6a, L.4-s or A.4-s.
This means that, so far as the appearance of strychnine-spikes in the ECG upon local strychninization of these areas is concerned, no functional boundary between the leg- and arm-subdivisions exhibits itself. Furthermore the local strychninization of either L.4-s or A.4-s results, as shown, in a suppression of the ECG of L.4 and A.4; thus, in this respect, also no boundary appears. These findings contrast with the functional boundaries between these subdivisions of the sensory cortex as they manifested themselves by the distribution of the symptoms of sensory excitation in the experiments on the monkey by one of us in 1924.
This contrast, however, is not a contradiction. The ECG expresses the activity of the cortex at that level of the CNS, the hypersensitivity depends also upon lower levels, notably the optic thalamus. Experiments in which the electrical activity of the thalamus, the electrothalamogram, was recorded before and after local strychninization of the sensory cortex have provided a satisfactory explanation for the apparent discrepancy mentioned above. The full discussion of this problem must, however, be reserved for a subsequent paper, which deals with the functional interrelation of the sensory cortex and the thalamus.
Our area 4-s lies as a narrow band of cortex in front of L.4 and A.4. The occipital border usually runs from the posterior end of the arcuate sulcus through the posterior portion of the superior precentral sulcus, and continues almost parallel to the central sulcus onto the medial aspect of the hemisphere. This border, however, is more or less arbitrary because of the great variability in size, form and direction of the superior precentral sulcus and of the arcuate sulcus. For the anterior border of area 4-s there is no landmark on the surface of the hemisphere. All we can say on the basis of our physiological observations is that area 4-s is a narrow band of cortex, the width of which measures ca. 2 mm. at its bottom and 3 to 4 mm. at its top.
It should be pointed out that extent and location of area 4-s, as given in Figure 1, should be regarded as an “average” of numerous experiments.
The variability in the pattern of the sulci of the hemisphere of Macaca mulatta (and other species) is so great that the location of area 4-s can only be “diagnosed” experimentally. This leads in many experiments to interesting composite, additive results. If the strychnine is applied not strictly within area 4-s, but encroaches upon area 4, this will show up as a combination of a suppression of the ECG in L.4 and A.4, the part of the symptomatology due to the strychninization of 4-s, with occasional, rather small spikes in L.4 or A.4, due to the strychninization of the most anterior part of this area. If the intended strychninization of area 4-s has been performed too much frontally, the most posterior portion of area 6a will also be involved. This will show up by the fact that together with the suppression of the ECG in area 4, there will appear small spikes in L.4 and A.4 and large spikes in area 6a. Only if the strychninization has been performed “purely” in area 4-s will a “pure” suppression in L.4 and A.4 appear without any trace of spikes in these areas or in area 6a. In fact, we have in several animals performed such a series of differently located local strychninizations in this region, thus establishing the exact location and extent of area 4-s in each specimen. Usually area 4-s was found to lie more forward than expected on the basis of examination of the surface configuration, especially on brains in which a large posterior “spur” of the arcuate sulcus is present.
The resemblance in extent and location of area 4-s with the “strip” of Marion Hines(8) is quite striking. Electrical stimulation of this strip of cortex in her hands abolished existing motor activities, extirpation resulted in spasticity, findings entirely in harmony with the temporary suppression of the ECG of L.4 and A.4 upon local strychninization of area 4-s. There can be, therefore, no doubt that the “strip” of Hines is identical with our area 4-s. Dr. Hines has written us that she dislikes the colloquial designation of this area as “the strip,” an expression having come into use as laboratory slang. The cytoarchitecture of this area, according to Dr. Hines, is similar to that of area 4, but can readily be distinguished from it by the great reduction in the number of Betz cells. It seems advisable and appropriate, therefore, to designate this area as area 4-s.
The cortex in front of area 4-s has the cytoarchitecture of area 6 of Brodmann. Dr. Hines wrote: “And if one is very fussy about cytoarchitecture this area 6 could be divided into two regions again. The pyramidal cells in layer III of the posterior part of area 6 are larger than those found in the same layer of the anterior part of this area.” So far we have not been able to find any functional differentiation upon local strychninization of area 6a, and have, therefore, refrained from subdividing it.
If it be permissible to transpose the cortex of Macaca mulatta upon that of cercopithecus of the Vogts (species not given so far as we know) it is obvious that our areas 4-s and 6a are not to be identified with the areas 6aα and 6aβ of C. and O. Vogt.
The suppression of the ECG of L.4 and A.4 by local strychninization of area 4-s is not brought about by cortico-cortical neurons. The experimental evidence upon which this statement rests, will be given in a subsequent paper. The observations presented in this paper confirm the hypothesis advanced by Dusser de Barenne in 1916 and again in 1924 to explain the finding that strychninization of a few square millimeters of the sensory cortex gives rise to symptoms of sensory excitation in a large portion of the body. At that time it was stated “as the most plausible explanation that the strychnine brings the small cortical area poisoned by it into a condition of abnormal and intense hyperexcitability and hyperactivity, and that this condition irradiates from the area poisoned over the whole of that part of the cortex which is in close functional connection with it” [(5) p. 285; see also (4) p. 383]. This assumption has now been proved to be correct in general. The present experiments have revealed details which have been dealt with in this paper.
The method of local strychninization with simple “clinical” observation of the animal failed to reveal functional differentiation within the sensory cortex except for the existence of functional boundaries between its major subdivisions, and, therefore, could not throw further light upon the problem of functional localization within the sensory cortex. The method of local strychninization was used in conjunction with recording of the ECG with the definite anticipation that this combination of methods would uncover just such finer differences of functional organization in the sensory cortex; this expectation has been confirmed by the experiments presented in this paper. These observations have permitted amplification and specification of the original hypothesis.
Summary
- The method of local strychninization in combination with that of recording the electrocorticogram (ECG) has proved suitable for study of the problem of functional organization within the sensory cortex of the monkey.
- Local strychninization of the cerebral cortex induces typical changes in the ECG, namely the temporary appearance of large and rapid potential fluctuations, or “strychnine-spikes."
- The distribution of these spikes differs with the locus of strychninization. In the visual cortex (area 17 of Brodmann) and in area 5 of the sensory cortex they are restricted to the area strychninized and its immediate vicinity. In the sensory cortex the spikes are widespread and their distribution specific for different areas.
- These specific differences in distribution, described in this paper, are the expression of directed relations between the various portions of the sensory cortex, of a functional organization within this region.
- The local strychninization of two areas of the sensory cortex (areas 4-s and 1) results in a temporary suppression of the ECG of areas L.4 and A.4, while “firing” other portions of the sensory cortex.
- Experiments with recording of the ECG and local electrical stimulation of various areas of the sensory cortex showed that the distribution of the propagated excitation, manifesting itself in the spread of an electrical after-discharge, was the same as the distribution of the spikes elicited by local strychninization of these same areas.
- The observations reported here confirm and amplify the original hypothesis of one of us of an intimate functional interrelation of the various areas within any one major subdivision of the sensory cortex.
Footnotes
References
Dusser de Barenne, J. G. Experimental researches on sensory localization in the cerebral cortex of the monkey. Proc. Roy. Soc., 1924, B 96: 272-291.
Dusser de Barenne, J. G. Experimentelle Untersuchungen uber die Lokalisation des sensiblen Rindengebietes im Grosshirn des Affen (Macacus). Dtsch. Z. Nervenheilk., 1924, 83: 273-299.
Brodmann, K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. III. Mitteilung. J. Psychol. Neurol., 1905, 4: 177-226.
Brodmann, K. Vergleichende Lokalisationslehre der Grosshirnrinde; Leipzig, J. A. Barth, 1909, v, 324 pp.
Vogt, C. and O. Allgemeine Ergebnisse unserer Hirnforschung. J. Psychol. Neurol., 1919, 25 (Erg. Heft 1): 277-461.
McCulloch, W. S. On the nature and distribution of factors for facilitation and extinction in the central nervous system. Amer. J. Physiol., 1937, 119: 363-364.
Dusser de Barenne, J. G. Die Strychninwirkung auf das Zentralnervensystem. IV. Mitteilung. Folia Neurobiol., 1912, 6: 277-286.
Hines, Marion. The "motor" cortex. Johns Hopk. Hosp. Bull., 1937, 60: 313-336.
Dusser de Barenne, J. G. Experimental researches on sensory localizations in the cerebral cortex. Quart. J. Exp. Physiol., 1916, 9: 355-390.
Epi-search analysis
Word cloud:
Activity, After-Discharge, Appear, Application, Area, Axons, Cell, Cerebral, Changes, Column, Cortex, Differs, Distribution, Ecg, Either, Electrical, Experiments, Figure, Findings, Fires, Found, Functional, Large, Leg, Local, Location, Locus, Method, Minutes, Observations, Paper, Portion, Present, Produces, Recording, Region, Relations, Results, Row, Sensory, Shows, Spikes, Stimulation, Strychnine-Spikes, Strychninization, Subdivision, Sulcus, Suppression, Surface, Various
Topics:
Area, Cortex, Strychninization, Spikes, Local, Strychnine, Sensory
Keywords:
Cortex, Strychnine, Strychninization, Post, Results, Activity, Deals, Spikes, Area, Organization (final)
Cortex, Ecg, Spikes, Stimulation, Experiments, Axons, Symptoms, Locus, Nerve, Sulcus (Full Text 1)
Cell, Cortex, Activity, Leg, Discharge, Fires, Ecg, Experiments, Results, Produces (full text2)
Citations
Related Articles
Related Books
Keywords from the citations and related material: Cortex, Brain, Monkey, Perception, Neuroscience, Analysis, Thalamus, Potentials, Rhesus, Rat